4. Nuclei and Radioactivity
1. This book is radioactive.
2. You are radioactive too, unless you have been dead for a long time.
3. The United States Bureau of Alcohol, Tobacco, and Firearms tests wine, gin, whisky, and vodka for radioactivity. If the product does not have sufficient radioactivity, it may not be legally sold in the United States.
4. Of those killed by the Hiroshima atomic bomb, the best estimate is that fewer than 2% died of radiation-induced cancer.
Those anecdotes are all true, and yet they surprise most people. That reflects the confusion and misinformation that pervades the public discussion of radioactivity. I hope that when you finish this chapter you can come back and read those four anecdotes and say, “Of course.”
Radioactivity
Radioactivity is the explosion of the nucleus of the atom. What makes this explosion so important and fascinating is the enormous energy released, typically a million times greater than in chemical explosions for the same number of atoms.
Atoms are small but not completely invisible. A device called a Scanning Tunneling Microscope (called an STM by experts) can pass over individual atoms, feel their shape, and then present that on a computer screen in the form of an image. A similar device can pick up individual atoms, carry them, and place them at new locations. In the photo below we show 35 xenon atoms arranged to form the letters “IBM” on the surface of a nickel crystal. (Guess what company did this work.)
|
|
“Visible”
atoms. The letters IBM were
written by arranging individual xenon atoms on the surface of a nickel
crystal. The atoms were manipulated and photographed using a
scanning-tunneling microscope. This work was done by a team led by Donald
Eigler. Guess which company they worked for. (Copyright IBM) |
It is this ability to manipulate individual atoms that has led to the excitement about the new field called “nanotechnology.” The name comes from the fact that an atom is about 1/10 of a nanometer (a billionth of a meter) in diameter.
To put the size of an atom in perspective, consider the following examples: a human hair has a thickness of about 200,000 atoms and a human red-blood cell has a diameter of about 10,000 atoms. These numbers are big, but not huge. I didn’t have to use scientific notation. So atoms are small, but they are not infinitesimally small.
Each atom consists of a cloud of electrons with a tiny nucleus in the center. The nucleus has a radius of about 10-13 cm, which means it is 100,000 times smaller than the atom itself. To visualize this ratio, imagine that an atom were enlarged until it was the size of a baseball or football stadium (300 m). Then the nucleus, similarly expanded, would only be the size of a mosquito (3 mm). Since its linear size is 10-5 times the size of the atom, then its volume is 10-15 times the volume of the atom (since you calculate volume by taking the cube of the linear size). That’s like the volume of the stadium compared to the volume of the mosquito. This enormous disparity often gives rise to the statement that the atom is mostly “empty space.” Some could argue, however, that the space isn’t really empty; it is filled with the electron wave. We’ll talk more about that in Chapter 10 “Quantum Physics.” Yet, even though it has only 10-15 of the volume of the atom, the nucleus contains more than 99.9% of the mass of the atom. The nucleus is very small, but very massive. That was not predicted; try to imagine the surprise and disbelief of scientists in 1911 when Ernest Rutherford discovered this incredible fact. It seems completely implausible. But it is true.
Within 20 years of Rutherford’s discovery, we learned that the nucleus itself was made up of even smaller pieces. The most important of these are protons and neutrons:
Protons weigh almost 2000 times as much as electrons and have the same magnitude of electric charge, but they are opposite in sign. (We’ll discuss the sign of the charge in Chapter 6. Electrons, by convention, have negative charge, and protons have positive charge.)
Neutrons are similar in mass than protons (they are actually about 0.3% heavier), but have no electric charge, i.e. they are “neutral”--hence their name.
So here is the basic picture of the atom: it has a very small nucleus made of protons and neutrons. Surrounding this is a relatively large volume occupied by electrons. But most of the mass is in the tiny nucleus. The nucleus of an atom weighs almost exactly the same as the entire atom itself since the electrons are so light.
Scientists love to deconstruct. So it is natural for them to wonder whether protons and neutrons are made of smaller objects. The answer was uncovered in the last few decades of the 20th century: protons and neutrons are made of particles called quarks[1] and a variable number of lightweight gluons that hold the quarks together (they are named after glue). We’ll discuss these further in an optional section at the end of the chapter. What are quarks made of? According to the unproven string theory, they (as well as electrons) are made of something called strings. I summarize this in the list on the next page.
Matter is made of molecules (e.g. water is made of H2O)
Molecules are made of atoms (e.g. H2O = hydrogen and oxygen)
Atoms are made of electrons orbiting a nucleus
Nuclei are made of protons, neutrons, and other light particles (e.g. gluons)
Protons and neutrons are made of quarks and gluons
Quarks and electrons may be made of strings
Elements and Isotopes
The number of protons in the nucleus is called the “atomic number.” This number also indicates the number of electrons orbiting the nucleus. An atom of hydrogen has 1 proton in the nucleus (and 1 electron in orbit), and we say it has atomic number 1. An atom of helium has 2 protons in the nucleus and 2 electrons in orbit. It has atomic number 2. An atom of uranium has 92 protons in the nucleus and 92 electrons in orbit. We say it has atomic number 92. Each element has a different atomic number. Here is a list of some of the atomic numbers of elements we will be discussing in this chapter:
|
Element |
Atomic Number (Np) |
|
hydrogen |
1 |
|
helium |
2 |
|
lithium |
3 |
|
carbon |
6 |
|
nitrogen |
7 |
|
uranium |
92 |
|
plutonium |
94 |
As mentioned earlier, the nucleus consists primarily of protons and neutrons. Neutrons don’t have electric charge, so they don’t change the behavior of the atom (at least, not much). But they do make the nucleus heavier. Atoms of an element with different numbers of neutrons are called different “isotopes” of that element.
For example, the nucleus of ordinary hydrogen (the abundant kind) always contains one proton and no neutrons. But about 1 in every 6,000 hydrogen atoms has a nucleus that contains an extra neutron. That kind of hydrogen is called deuterium, or “heavy hydrogen.” Water made from heavy hydrogen weighs more; it is called heavy water. Heavy water was very important during World War II in the development of the nuclear reactor. In fact, Hitler had a special plant to purify deuterium (useful to make a nuclear reactor), and the Allies sent a team to blow that plant up.
About 10-18, or a billionth of a billionth, of ordinary hydrogen has 2 neutrons in the nucleus. This kind of extra-heavy hydrogen is called tritium. Tritium is the only kind of hydrogen that is radioactive. It is used in medicine and in hydrogen bombs.
We’ll be talking a lot about deuterium and tritium, especially when we get to nuclear reactors and bombs. So learn those terms. Here are useful memory tricks:
In deuterium the proton and neutron in the nucleus form a duo.
In tritium the proton and neutrons in the nucleus form a trio.
Over 99% of uranium found in the Earth has a nucleus with 92 protons and 146 neutrons, making up a total of 92+146 = 238 particles in the nucleus. This is called U-238. But about 0.7% of the uranium has only 143 neutrons in the nucleus instead of 146. This is a different “isotope” of uranium called U-235. It is very important, because U-235 plays a key role in the atomic bomb and nuclear reactors.
Both U-238 and U-235 have 92 protons. That means they both have 92 electrons. Since it is the electrons that play the major role in ordinary chemistry, both isotopes react very similarly with other elements, such as oxygen and water. That’s why they are both called uranium. But when we are interested in the properties of the nucleus, particularly in nuclear explosions, then the difference in neutrons becomes extremely important.
Radiation and Rays
Now let’s return to the radioactivity, the explosion of the nucleus. A common chemical explosion (e.g. TNT) takes place when a large molecule suddenly breaks up into smaller molecules. In a similar way, a radioactive explosion takes place when a nucleus breaks up into smaller parts.
We’ll begin with the most common type of radioactivity, in which a relatively small particle is thrown out from a big nucleus. It flies out like a bullet, at very high speed, sometimes approaching the speed of light. When this process was first discovered, nobody knew what was coming out. The projectiles couldn’t be seen directly, but they passed through matter and could expose photographic film. The projectiles were called “rays,” probably because they travel in nearly straight lines. They had properties similar to X-rays, which Wilhelm Conrad Roentgen had discovered a few years earlier in 1895. Different kinds of rays were found, with somewhat different properties, and they were named after the letters of the Greek alphabet.[2] Some rays (e.g. from uranium) could be stopped by a piece of paper; these were called alpha rays. Rays with more penetration were called beta rays. The most penetrating of all were called gamma rays. (There were delta rays too--but those turned out to be the same as low energy beta rays so the term is little used.)
The old terminology has changed; instead of saying rays, we now say radiation. It is worthwhile to learn this formal terminology:
radioactivity refers to the explosions of atomic nuclei
radiation consists of the pieces that get thrown out in the explosion
Each ray (or particle) is like a tiny bullet, so tiny that you don’t feel it if it hits your body. Alpha rays and beta rays bounce off many atoms before they stop; with each bounce, they can knock apart a molecule or mutate a gene. The slowing bullet leaves a trail of damaged molecules along its wake. The damage is small but, if you are hit by a large number of particles, the total effect can make you ill or even kill you. Gamma rays tend to be absorbed by a single atom; however, they frequently break up the atom or even its nucleus, so secondary radiation is emitted. It is that secondary radiation that often does the most damage.
“Seeing” radiation
– the Cloud Chamber
When alpha or beta rays pass through a gas, they knock electrons off atoms, creating a trail of charged particles called ions. If the gas has a lot of water vapor or alcohol vapor mixed in, and the gas is cool, then the water or alcohol tends to form little droplets on these ions. In essence, they form clouds along the path of the radiation. White tracks suddenly appear when an alpha or beta ray passes through a chamber set up in this way. In the image below, we show some images taken in the original cloud chamber, invented by Charles Wilson (for which he got the Nobel Prize in 1927). The streaks are the cloud particles along the path of the radiation.
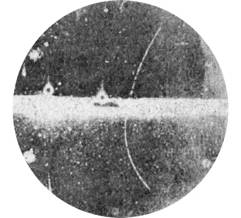
Cloud Chamber with antimatter
The thin curved line is a trail
of cloud particles left behind by a positron. This image was the first antimatter ever observed, and it
won Carl Anderson a Nobel Prize. The horizontal broad white region was a
divider that the positron passed through.
(Department of Energy photo)
The tracks are very similar in nature to the vapor trails left behind when a jet airplane engine passes overhead. The track doesn’t actually show the airplane itself, but it shows where it has passed.
A cloud chamber is a marvelous thing to watch. Radiation from a radioactive source will show short white lines of cloud particles suddenly forming along a path. The droplets are heavy, so they drift to the bottom of the chamber. Meanwhile, new paths are suddenly appearing above them. Every once in a while, radiation all the way from space will cause a long path to appear in the cloud chamber. This radiation is known as cosmic radiation.
Radiation and Death: the rem
The biological damage done to cells hit by radiation is measured in a unit called the rem. I can give you a rough idea of how big a rem is from the following example. Suppose each square centimeter of your body is penetrated by 2 billion gamma rays. If that happens, then the radiation dose to that part of your body is approximately 1 rem.[3] A rem usually refers to the amount of damage to each gram of your body. If your whole body is exposed, then we say you got a whole body dose of 1 rem. It means that each gram of your whole body suffered the same damage.
Two billion gamma rays sounds like a lot of radiation, so it might make you think that a rem is a huge amount of damage. But remember, the nucleus is very small. When it emits energy, the energy is big only in a relative sense. For example, the gamma rays entering your body will deposit their energy, and that will cause your body to warm up. But the amount of warming can be calculated; it is less than 1 billionth of a degree C.[4] The radiation does damage individual molecules, and that is the source of all the real danger. Most of the time, the damage can be repaired by the cells in your body. But your body is not always successful. Many people estimate that the exposure to this much radiation (1 rem to every cell in your body, i.e. 1 rem whole body) will increase your chance of getting cancer by about 0.0004, or 0.04%. We’ll talk more about that shortly.[5]
The term rem was originally an acronym.[6] Physicists will never miss a chance to honor one of their own, so it was inevitable that a new unit would be introduced, the Sievert.[7] The conversion is simple: there are 100 rem in 1 Sievert. If you look at modern textbooks, you’ll see Sieverts used more and more often. But most public reports still persist in using the rem, so we will too. (Memory trick: the Sievert is capitalized and it is the big unit. It consists of 100 of the smaller, uncapitalized rem.)
Radiation poisoning
If every cubic centimeter in your body is exposed to 1 rem, then we say you have received a “whole-body dose” of 1 rem. If the whole-body dose is more than 100 rem, the damage done to the molecules of the cells is enough to disrupt the metabolism of the body and the victim becomes sick. This is called radiation poisoning. If you know someone who has undergone radiation therapy (to kill a cancer), then you know what the symptoms of mild radiation sickness are: nausea, listlessness (popularly referred to as “loss of energy”), and loss of hair. The severity of the illness depends very sensitively on the dose, as the table on the next page shows.
|
Whole-Body Dose |
Resulting Radiation Illness |
|
below 100 rem (below 1 Sievert) |
no short-term illness |
|
100 to 200 rem (1 to 2 Sievert) |
slight or no short-term illness;
nausea, loss of hair; rarely fatal |
|
300 rem (3 Sieverts) |
50% chance of death, if untreated
within 60 days |
|
more than 1000 rem (more than 10
Sieverts) |
incapacitation within 1 or 2
hours, survival unlikely |
In medical terminology, 300 rem is said to be the “LD50”--which stands for the lethal dose that will kill 50% of those exposed. Memorize the following:
LD50 for radiation is 300 rem = 3 Sieverts
You will frequently hear the term millirem being used when people talk about radiation leakage. One millirem is 1 thousandth of a rem. LD50 for radiation is 300,000 millirem. The reason that I want you to memorize these numbers is that you will very likely encounter radiation in your life, maybe at a doctor’s office, maybe elsewhere. You’ll probably hear the term millirem, rather than rem. A dental x-ray gives, typically, a few millirem to your jaw (not to your whole body). It is useful to understand how small a millirem really is.
Radiation and Cancer
Get ready for a paradox: the average dose that it takes to induce cancer is a whole body dose of approximately 2500 rem.[8] But a dose of 1000 rem will kill you within a few hours from radiation illness. So how can anyone get cancer from radiation? You might (incorrectly) think that the victim would die first. The solution to this paradox is found in the linear hypothesis.
The “Linear Hypothesis”
The solution to the paradox lies in the “linear hypothesis.” We can’t give 2500 rem to a single person without immediate death. But if we spread it out among 2500 people, each of whom will be exposed to 1 rem, then the same total damage will be done. Not one person will get radiation illness, but the same number of mutations will be induced--just spread out over many people. The linear hypothesis is a fancy name for the belief that the 2500 rem, even though distributed over a large number of people, will still cause 1 cancer--not 1 cancer each, but 1 cancer total among the 2500 people.
The
basis for belief in the linear hypothesis is the fact that most mutations are
harmless; they may cause the cell to die, but we have lots of cells, and many
of the cells can be replaced. But after 2500 rem of damage, the chances are
good that a particularly bad mutation will be created in one of the victims,[9]
a mutation that causes cancerous multiplication of cells.
Not all experts believe the linear hypothesis. They argue that the cell can repair minor damage, so if spread out enough, every body will be able to recover. We know that the linear hypothesis doesn’t work for radiation sickness. Although 1,000 rem will cause fatal radiation illness, 1 rem per person, spread over 1000 people, will cause no radiation illness whatsoever. Moreover, the linear hypothesis doesn’t work for most other kinds of poisons, such as arsenic.
Others counter by saying that radiation sickness, like other symptoms of poisoning, is different from cancer. Radiation illness occurs when the radiation damage overwhelms the body’s ability to recover. Cancer appears to be a much more probabilistic illness. You develop cancer when you get, by chance, exactly the worst possible kinds of mutations, creating cells that grow and divide and will not be turned off by normal bodily controls.
In fact, we don’t really know if the linear hypothesis is valid at low levels (e.g. 1 rem) of radiation exposure. That’s because cancer is a common disease. Even without radiation, 20% of people die from cancer. So with 2500 people, you expect 500 cancers anyway, even with no exposure to radiation.
Now let’s look at what will happen if each of the 2500 people is exposed to 1 rem. According to linear hypothesis, we expect one additional cancer in the group, i.e. 501 cancers. Statistical fluctuations make such a small effect virtually impossible to verify. Even with large numbers of people exposed (e.g. in the Chernobyl nuclear reactor accident; see the next section) the effect tends to be obscured by statistical fluctuations and systematic uncertainties.
But a premature death from cancer is a tragedy for anyone, even if it doesn’t appear in the statistics. One additional cancer is significant (especially to the person affected), even if it is not “statistically significant.” That’s why many people think we should assume the linear hypothesis, even if it is experimentally unverified, and use it as a basis for public policy. Since the linear hypothesis forms the basis for much public discussion, it is important for future presidents to know what it implies. It is equally important to know that it may not be accurate.
The Chernobyl Disaster
In 1986, the Chernobyl nuclear power plant in Ukraine had a violent accident. There was an explosion in the vessel that contained the radioactive fuel, and a huge amount of radioactivity was released into the atmosphere. We’ll discuss the innards of nuclear reactors in coming chapters; for now, all you need to know is that a vast amount of radioactive material was released. Several of the firefighters who put out the Chernobyl blaze died from radiation sickness. Radioactivity from the plant was carried by wind over populated areas. This was one of the biggest news items of the 1980s; everybody who was an adult at that time remembers it. There was fear around the world as the radioactive plume drifted. Some radiation from this event was detected in the United States.
The Chernobyl event was one of the most famous and important events of the entire decade. It was in the newspapers for months. It is cited today by many people, those who oppose the further use of radioactive processes (such as nuclear power), and by those who are in favor of nuclear power.[10] People use Chernobyl data to support their point of view, regardless of what that point of view is. So it is important to understand this event. It would be worthwhile to read more about the Chernobyl accident. You will find many links to the Chernobyl disaster on the Internet.
 Even
though a lot of effort went into mapping the spread of the radiation, most of
the damage was done in the first few days. So it is hard to know the total
radiation exposure to humans. The firemen who died received doses of several
hundreds of rem. Initial estimates were that between 25,000 and 40,000 people
had received a median dose of 45 rem. The government decided to evacuate all
regions in which a person would receive a lifetime dose of 35 rem or more. The
nearby city of Chernobyl fell below this limit. The cloud of debris blew over
1,000 miles, towards Stockholm Sweden, giving most people in that city a dose
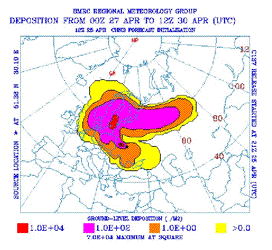
exceeding 1 rem. A map of the areas most severely exposed is shown in the
figure on the right. An initial
estimate was that the total dose to the world population was 60,000,000 rem.
This number is obtained by estimating the number of rem received by each person
on the Earth and then adding those numbers together.
Even
though a lot of effort went into mapping the spread of the radiation, most of
the damage was done in the first few days. So it is hard to know the total
radiation exposure to humans. The firemen who died received doses of several
hundreds of rem. Initial estimates were that between 25,000 and 40,000 people
had received a median dose of 45 rem. The government decided to evacuate all
regions in which a person would receive a lifetime dose of 35 rem or more. The
nearby city of Chernobyl fell below this limit. The cloud of debris blew over
1,000 miles, towards Stockholm Sweden, giving most people in that city a dose
exceeding 1 rem. A map of the areas most severely exposed is shown in the
figure on the right. An initial
estimate was that the total dose to the world population was 60,000,000 rem.
This number is obtained by estimating the number of rem received by each person
on the Earth and then adding those numbers together.
 If we assume that the linear
hypothesis is correct, we can now calculate the number of cancers induced in a
remarkably simple way: just divide the number of rem by 2500 to get
60,000,000/2500 = 24,000 cancers world-wide. This number was widely publicized. But it was probably wrong.
If we assume that the linear
hypothesis is correct, we can now calculate the number of cancers induced in a
remarkably simple way: just divide the number of rem by 2500 to get
60,000,000/2500 = 24,000 cancers world-wide. This number was widely publicized. But it was probably wrong.
The radiation fallout has to be known accurately with respect to the population distribution. If you are indoors when the radioactive particles fall, you don’t breathe them in. Short-lived radioactive particles, if they remain at high altitude and decay, then don’t contribute to the rems absorbed by people. Because of these factors, the estimate of the dose from Chernobyl is constantly being revised as better estimates are made, and that will continue into the future. In 2006, the International Atomic Energy Agency (the “IAEA”, associated with the United Nations) estimated that the total number of cancer deaths from the Chernobyl accident would total 4,000, not 24,000. Several groups hotly disputed this revision. There was even a counter-estimate made by an anti-nuclear group saying the number of deaths was actually 500,000. I believe this estimate was made by looking at death rates in the Chernobyl region, and attributing all excess deaths to the accident. That is not an accurate way to do it.
Who is right? That requires a careful look at the way the data were analyzed. Some people will tend to believe the IAEA, because they have established a good reputation with past scientific work. Others will distrust them, because the people who did the work are scientists, many of whom have worked for organizations such as the Department of Energy (formerly the Atomic Energy Commission), and are therefore suspected of pro-nuke bias. But they made the best scientific estimate, so I think the 4,000 figure is probably most accurate. It doesn’t really matter for the purposes of illustration. 4,000 is a very large number of deaths.
Now let’s calculate the expected cancers for the people near the nuclear plant. Based on the numbers given above, assume 40,000 people received 45 rem on average.[11] Multiply these to get the total of 1.8 million rem. Divide by 2500 to find the total number of cancers expected, equal to 720. (These people were already counted in the 4,000 number.)
This is clearly a great tragedy. And yet, a surprising fact for many people is that even if this calculation is correct, and the number of cancers predicted is accurate, it will be difficult to identify the people who are being killed by the Chernobyl accident. There are too many other cancers.
Let’s consider the nearby victims. Among 40,000 people, we would expect 20% to die of cancer anyway. That means 8,000 deaths that are not a result of the radiation. On top of these, we predict another 720 deaths from the radiation exposure. So the total cancers, instead of being the “normal” 8,000, will rise to 8720. Of these 8720, only 8% were caused by the Chernobyl accident. It is difficult to know who is dying from “ordinary” cancer and who is dying from the radiation effects, unless the kinds of cancer induced by radiation are different. (There are some differences: thyroid cancer is more prevalent, for example, from radiation—although it rarely leads to death.)
In the population of 100 million people who received measurable radiation, we expect there to be approximately 20 million cancers from other causes. Let’s pretend for a moment that this number is exact: 20,000,000 “ordinary” cancers. Because of Chernobyl, this number will be increased to 20 million + 4 thousand = 20, 004,000. Put another way, the probability of anybody getting cancer, if exposed, has been increased from 20% to 20.004%. Given the natural variations in cancer, most people think that we will not be able to see the increase statistically.
Yet those 4 thousand people are all individuals who otherwise would not have died from cancer. There is a strange paradox here: tragedy is occurring, and yet is almost invisible among the much larger tragedy of cancer from other causes.
Cancer from the nuclear bombing of Hiroshima
I stated in the opening paragraphs of this chapter that, in Hiroshima, fewer than 2% of the deaths caused by the atomic bomb were from cancer. The reason is simple: unless you were far from the center of the blast, you were killed by the blast or fire. Still, some who survived the fireball received enough radiation to die of radiation sickness. The best estimate is that there were about 52,000 survivors (not killed by those other effects) who received 0.5 rem or more; the average dose for these people was 20 rem. That means that the total dose was 52,000 x 20 = 1,040,000 rem. Divide this by 2500 rem per cancer, to get the total expected number of cancers is 1,040,000/2500 = 416. That is 0.8% of the 52,000 people.
Estimates of the total number of people killed by the Hiroshima bomb vary between 50,000 and 150,000. (It is hard to know how many people were there when the destruction was so total in the core of the city.) But you can see now why the cancer deaths were less than 2% of this.
Cancer deaths could have been higher if the bomb had been exploded when it was close to the ground, rather than at high altitude, because that would have increased the amount of radioactive “fallout.” We’ll discuss fallout further in Chapter 5.
High radiation in Denver
Denver is located in a geologic area that emits (from the ground) higher-than-average amounts of the radioactive gas radon. A reasonable estimate[12] is that the average yearly excess in Denver (compared to the US average) is about 0.1 rem per person per year. For 2.4 million people living in Denver for 50 years, this excess amounts to (0.1)(2.4 x106)(50) = 107 rem, which should cause 4800 excess cancers.
So here is another paradox: the actual cancer rate in Denver is lower than the average in the US. How can that be? Does it mean that the linear hypothesis is wrong? Or could there be other effects, even more important than radioactivity, that cause cancer? Can you guess what they are? Do you expect that there would be differences in lifestyle, eating habits, exposure to sunlight and ultra-violet radiation, or genes? Anything else? Does that mean that someone who moves to Denver lowers his risk of cancer?
In case you are hoping I can give you the answer, I can’t. Nobody knows the answer. But there are lots of possible explanations. In the meantime, I would not consider the excess radiation of Denver to be an important factor in a decision to live there.
However, before you buy a house in the Denver area, you might want to measure the radon level to make sure that you are not buying one of the highly radioactive ones. This is a serious comment; some houses have been measured to have dangerous levels.[13] The Environmental Protection Agency offers guidelines for making such measurements, and commercial devices are available.
Tooth and chest x-rays
You are exposed to radiation every time you get an x-ray photograph taken of you. Such photographs are now called “x-rays” themselves, but that is just short for “x-ray photograph” or “x-ray image.” You may have noticed that the person who takes the image leaves the room when the x-ray machine is turned on. For a tooth x-ray, a lead shield may be placed over you body to protect your “vital organs.” This frightens many people. What is the danger?
Next time an x-ray is taken, ask the technician for the dose in rem. (Odds are, the technician won’t know, but will simply assure you it is safe.) A typical dose for a dental x-ray is less than 1 millirem (i.e. 0.001 rem); for the purpose of the calculation, let’s assume the dose is 1 millirem = 1 mr = 10-3 rem.
The numbers that we gave for cancer assumed a “whole-body dose.” That means, they gave the cancer rate assuming that every part of your body got the same dose. But when your tooth and jaw are exposed, it is probably not more than a pound of flesh that is exposed. Let’s assume it is 1% of your body. Then, by the linear hypothesis, such an exposure is only 1% as dangerous as a whole-body dose. That makes it equivalent to 1% of a millirem whole-body, i.e. 10-5 rem. From the linear hypothesis, it takes 2500 rem per cancer. That would require 2500/10-5 = 250,000,000 = 250 million tooth x-rays. Put another way, one tooth x-ray will induce cancer with the chance of 1 in 250 million = 1/250,000,000 = 4 x10-9.
Let’s now calculate the risk of cancer from a chest x-ray. Modern chest x-rays are about 25 mrem to about 50 lb of your body. That is a much greater dose than you get from a tooth x-ray. It is 25 times more millirem to 50 times more body, which means the dose is 25x50 = 1250 times greater. The number of cancers per chest x-ray should also be 1250 times greater than for the tooth x-ray. That number comes out to be 1250 x 4 x10-9 = 5 x10-6.
X-rays and pregnancy
Radiation can be particularly dangerous to the fetus. Mutation in one of the stem cells (cells that can turn into other cells) can lead to mental retardation, malformed growth, or cancer. If the mother has a tooth x-ray, or one to her ankle, then the danger is tiny.
As with other low-dose effects, our actual knowledge of the effects of x-rays is limited; it is based primarily on high-level exposures (e.g. in accidents and WWII) and the linear hypothesis. The United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) has studied this problem, and concluded that the risk to the fetus is about 3% for each rem of exposure.
If a dental x-ray were applied directly to the fetus, the linear hypothesis says that the 1 mrem dose would result in a risk 1000 times smaller, i.e. 0.003% for each mrem. If the radiation is delivered to the tooth, and the only radiation reaching the fetus is 100 times smaller, then the risk goes down by a similar factor. It is possible that the dangers of the mother’s untreated tooth can be greater to the fetus than these small amounts of radiation.
Ultrasound
Many pregnant women are exposed to ultrasound. Although technically ultrasound is a form of “radiation” (as is sound itself), it does not have any of the ability to cause mutation that x-rays or beta rays have. Ultrasound is high-frequency sound, and it does not have the capability of delivering enough energy to individual cells to cause mutations.
Ultrasound could have other negative effects, but ultrasound radiation should not be confused with nuclear radiation. We’ll talk more about it in Chapter 9.
Radiation to cure cancer
This may seem paradoxical, but one of the most effective methods to cure cancer uses radiation. Cancers are not healthy cells; they have been mutated to absorb nourishment and divide rapidly. Because they are specialized for division rather than long life, many cancer cells are more vulnerable to radiation poisoning than are healthy cells. So a common way to treat cancer is to hit it with high levels of radiation.
This is typically done by aiming the radiation right at the cancer. The radiation can enter the body from many directions, but it is focused on the cancer. The focusing is designed to make sure the cancer cells get a higher dose than do the surrounding cells. Cells in the vicinity of the cancer also have their metabolism disrupted, and that can induce radiation illness. The goal is to kill the less resistant cancer cells; other cells get sick, but recover. The difficulty, as with all cancer treatment, is that it is necessary to kill virtually all the cancer cells in order to prevent the cancer from returning.
Radiation treatment is often combined with chemotherapy (cancer cells are also less resistant to poisons) so you cannot always tell which effects are due to which treatments. Maybe for this reason radiation treatment is often confused with chemotherapy. Don’t confuse them.
Some people avoid radiation treatment because they fear the side effects. Others avoid it because they fear that the radiation will induce cancer. That argument does not make numerical sense. Compare the tiny risk of an additional induced cancer to the extreme danger of the cancer that is already present. But the fear of radiation is so great that some doctors cannot convince the cancer patient to get this treatment.
Dirty Bombs
Can a terrorist explode a tank of radioactive material in the middle of a city, and make it uninhabitable for the foreseeable future? A device that releases large amounts of radioactivity is called a “dirty bomb” or a “radiological weapon”. This scenario has received a lot of attention in the news. Yet it is harder to do this than you might think.
Assume, for the moment, that you are a terrorist and you are designing such a weapon. You want to spread radioactive material over a square kilometer. Your goal is to make the area so dangerous that people would get radiation illness if they were exposed for 1 hr. Let’s say the bomb will be roughly 1 m in size and will be delivered by a truck. To do this you will have to concentrate the radioactive material by a factor of 1 million (since 1 km2 contains 1 million m2). That means someone within 1 m of the bomb will get a lethal dose in 1 millionth of an hour, i.e. in 3.6 milliseconds. To avoid that, the person might try to stay 10 ft (3 m) from the bomb. But that distance would still give an LD50 dose in 36 milliseconds.[14]
To avoid this, the terrorist might try shielding the radiation with lead. A half-centimeter shield of lead will reduce the radiation by a factor of 3 – that’s not very much. A full-centimeter shield will reduce it by 9, and a 2-centimeter shield will reduce it by 81. Let’s try that. The weight of a 1-meter cube with lead walls 2 cm thick is about 1.6 tons. You could carry that in a truck. It would still give an LD50 dose, at a distance of 10 m, in 3 sec. That doesn’t mean that a radiological weapon is impossible. It just means that it is much harder than many people suppose.
Jose Padilla was a Chicago street thug recruited by al Qaeda to build and detonate a radiological bomb in the United States. (I recommend a Google search on his name.) He was captured by the police before he made any definite plans. But in the deposition for his trial we learned that al Qaeda had told him to abandon plans for a radiological weapon, and instead use natural gas to blow up some apartment buildings. I think it is likely al Qaeda realized that a working radiological weapon was essentially beyond the means of terrorists, and instead are likely planning to use again the method that devastated the World Trade Center--exploding fossil fuel.
For an essay I wrote on radiological weapons, see The Dirty Bomb Distraction at www.muller.lbl.gov/TRessays/29-Dirty_Bombs.htm
What Are the Rays, Really?
It took many years to figure out what the rays really were. Here are the answers:
Alpha rays are chunks consisting of 2 protons and 2 neutrons moving at a high velocity. This is identical to the nucleus of a helium atom. Because we know these rays are actually particles, they are sometimes referred to as “alpha particles,” or simply “alphas.” When alpha rays finally slow down, they usually attract 2 electrons (either free electrons or ones that were weakly attached to atoms) and form a helium atom.
Beta rays are energetic electrons. They are much lighter than particles like alphas, yet they move so fast that they have energy comparable to that of the slower alpha particles. When betas finally stop (after numerous collisions), they usually attach themselves to an atom.
Gamma rays are packets of very energetic light. Gammas travel at the same speed as light (3 x108 m/sec), but they typically carry a million times as much energy as a single packet of visible light. (We’ll talk about light packets, also called “photons,” in Chapter 10.)
Neutrons are massive particles like protons, but with no electric charge. Neutron emission is very important in the chain reaction, which we will discuss in Chapter 5. The neutron bomb is a nuclear weapon that emits a large number of neutrons. Its purpose is to induce radiation illness in people, while doing relatively little damage to buildings. The ethics of such weapons were hotly debated in the 1970s.
X-rays are the most famous of the radiations, since they have become so important in medicine and dentistry. They are packets of light, like gamma rays, but a factor of 10 to 100 times less energetic. They pass through many materials (such as water and carbon), but are quickly stopped by elements with a high atomic number, such as calcium and lead. Because x-rays are stopped by calcium, this allows them to be used to look at tooth decay and broken bones. An “x-ray” photograph is actually a shadow of the calcium projected on a piece of film. The character “Superman” could not use his x-ray vision to look through lead, since it is one of the heaviest elements, and therefore absorbs x-rays.[15] Lead is used to protect from x-rays. When you get a tooth x-ray, the dentist will likely put a lead-filled apron over other parts of your body to make sure that scattered x-rays don’t reach you. (They actually use far far more lead than necessary. They have discovered that people feel more comfortable about getting x-rays if they feel a heavy blanket on them.)
Cosmic rays are any radiation that comes from space. Cosmic rays consist of protons, electrons, gamma rays, x-rays, and muons--unusual particles that can pass through a hundred meters of rock, and are themselves radioactive. Because of their penetrating power, they have been used to x-ray the Egyptian pyramids.
Fission fragments are a particularly dangerous kind of radiation that is emitted when a nucleus undergoes fission, i.e. it splits into two or more pieces. Fission fragments are chunks containing large numbers of protons and neutrons, and they are themselves usually highly radioactive. Their real danger comes from when they stop and then re-decay. These are the radioactive particles that make fallout from nuclear bombs so dangerous.
Cathode rays were discovered emanating from hot metal that had a high voltage applied. It wasn’t known that the rays are actually electrons; the electron hadn’t been discovered yet. That had to wait for the 1897 work of J.J. Thomson. Most people still call the device that uses these beams “cathode ray tubes” or “CRTs.” As of the early 21st century, many TV and computer screens are still made of CRTs, but they are quickly disappearing. If you’re young, your children may never hear this term, since in the near future all CRTs will likely be replaced with thin screens. For more on the history, see: www.aip.org/history/electron/jjrays.htm
Neutrinos are the most mysterious of all the rays. They are usually emitted at the same time that beta rays (electrons) are emitted from a nucleus. Neutrinos have such small mass that, even when they have only moderate energy, they move nearly at the speed of light. They do not “feel” electric or nuclear forces, so they pass through the entire Earth with only a small chance of hitting anything. They are so strongly emitted by the sun, over 1010 of them are passing through every square centimeter of your body every second. Nevertheless, they are the least dangerous radiation you are exposed to.
Radiation from cell phones occurs in the form of microwaves, which are very low energy packets of light, even lower energy than those of visible light. Microwaves deposit their energy in the form of heat; that’s why they can be used in microwave ovens. Microwaves do not break DNA molecules in the body, and therefore they pose no risk of causing cancer in the way that alpha, beta, gamma rays, and even sunlight can. Much of the fear of microwaves comes from the fact that they share the name “radiation” with the other, far more dangerous forms such as gamma radiation.
You Are Radioactive
A typical human body contains approximately 40 g of potassium. Most of this is the stable, non-radioactive isotope potassium-39. Each nucleus of potassium-39 contains 19 protons and 20 neutrons, totaling 39 (that’s why it is called potassium-39). But about 0.01% of the potassium atoms have an extra neutron in their nucleus, these are called potassium-40. Potassium-40 is radioactive. This means that your body contains 40/10,000 = 0.004 g = 4 mg of a radioactive cancer-producing isotope. The number of radioactive potassium-40 atoms in your body is 6 x1019. This is not an artificial radioactivity, but it is left over from the formation of potassium in the supernova that gave birth to our solar system (more on this later).
Potassium-40 is often abbreviated as “K-40.” The K comes from the Latin name “kalium” for pot ashes--the original source of potassium. Parts of the word kalium also survive in the word “alkali.”
Approximately 1,000 atoms of K-40 (read this aloud as “potassium-40”) explode in your body every second. Your body is radioactive. About 90% of the explosions produce an energetic electron (beta ray); most of the rest produce an energetic gamma ray. So there are about 1,000 self-inflicted radiations per second from your own body. This radioactivity within your body produces a dose of approximately 0.016 rem = 16 millirem over a 50-year period. If the linear hypothesis is correct, we calculate the cancer induced by dividing the rem by 2500. Your chance of having a self-induced cancer is 0.016/2500 = 6.4 x10-6, i.e. about 6 chances in a million. That’s small, although it is higher than your chances of winning a typical grand lottery.
The results are more interesting if you think about the consequences for a large population. There are about 300 million people in the United States. Multiply 300 million by 6 millionths of a cancer per person, and you find that 300 x 6 = 1800 people will die of cancer over the next 50 years in the United States, induced by their own radioactivity. That averages to 36 per year in the US. If you sleep near to somebody, then their radioactivity can affect you (see the discussion topic at the end of the chapter).
A second source of radioactivity in our bodies comes from carbon-14, also called “radiocarbon,” and abbreviated C-14. The C-14 nucleus is similar to that of the ordinary C-12 nucleus, except that it has two extra neutrons (increasing the atomic weight from 12 to 14). But, it turns out, those extra neutrons make carbon-14 radioactive. In carbon-14, one of the neutrons will explode, emitting an electron and a particle called a neutrino (which we’ll describe in just a moment). When the electron and neutrino are emitted, the neutron turns into a proton, so the remaining nucleus is nitrogen. On average, half of the carbon-14 atoms in your body will explode in 5730 years. That period of 5730 years is called the “half-life” of C-14.
Every gram of carbon in your body has 12 atoms of carbon-14 exploding every minute. That is equivalent to 1 explosion every 5 seconds, on average. In an average body, there are about 3,000 such radioactive explosions every second.[16] This is in addition to the 1,000 K-40 decays mentioned earlier.
Now here is the really fascinating thing about C-14: we can use it to measure how long things have been dead. To see how this works, we have to understand a very strange phenomenon in radioactive decay that is called the “half-life rule.”
The Mysterious Half-Life Rule
As mentioned above, half the atoms of C-14 decay in 5730 years. It is natural to assume that the remainder would decay in the next 5730 years, but that is not what happens. Only half of them decay in the subsequent 5730 years. And then in the following 5730 years, only half of the remainder will explode. The remaining fractions after various years are shown in the table below.
|
Age of C-14
(yr) |
Number
of Half Lives |
Fraction
Remaining |
|
5730 |
1 |
1/2 |
|
11460 |
2 |
1/4 |
|
17190 |
3 |
1/8 |
|
22920 |
4 |
1/16 |
|
5730xN |
N |
1/2N |
The value of the half-life is different for different radioactive isotopes, but the behavior is similar. For K-40 the half-life is 1.25 billion years. In 1.25 billion years, half of the K-40 decays. In the next 1.25 billion years, half of the remaining atoms decay. There have been nearly 4 half-lives since the Earth was formed about 4.6 billion years ago. That’s why there is so much K-40 left; since the formation of the Earth, there hasn’t been enough time for all the K-40 to decay.
By the way, did you notice how similar this rule is to the rule we used to calculate the density of air at different altitudes (Page 3-23)? Recall that at an altitude of 18,000 ft, the density of air is half of what it is at sea level. Go up another 18,000 ft, and the density is reduced by another factor of 1/2, and so forth.[17] At 180,000 feet (10 such steps) the density is (1/2)10. The math is identical to the formula for radioactive half lives!
Radioactive Decay
If you have a large number of radioactive atoms, half of them will explode in one half-life. (It may not be exactly half, since the rule is based on probabilities.) Thus, one half-life later, there are only half as many radioactive atoms. That means that the number of radioactive explosions per second will only be half as great as it was in the beginning. This reduction of radiation was originally called “radioactive decay.” But now the word decay is applied much more universally. Physicists typically talk about a single nucleus undergoing radioactive decay. In this context, that word is used far more commonly than the word “explosion.”
Nuclei Die, But They Do Not Age
The half-life rule is true for all the known kinds of radioactivity. But the more you think about it, the more mysterious it is. People don’t die following the half-life rule. When we are born (at least in the U.S.), we are expected to live about 80 years. If we make it to age 80, we don’t expect to live another 80 years--yet that is the way it would work if our physical aging followed the half-life rule.[18] Please appreciate how strange the half-life behavior is! It is as if the atom does not age. The old carbon-14 is identical to the young one. No matter how old it is, its expected half-life is still 5730 years.
We don’t really understand this phenomenon, but physicists sometimes “explain” it by saying that radioactive decay is determined by the laws of quantum mechanics, which are probabilistic laws. For K-40, we say that the probability of decaying in 1.25 billion years is 50%. This probability doesn’t change, so no matter how old the atom is, its probability of decay remains 50% for the next 1.25 billion years. Of course, we haven’t really explained anything, because we don’t know why the laws of physics should be probability laws.
Table: The Half Lives of Some Important
Isotopes
|
Polonium-2l5 --- 0.00l8 seconds Polonium-2l6 --- 0.l6 seconds Bismuth-2l2 --- 60.6 minutes Sodium-24 --- l5.0 hours Iodine-l3l --- 8.l4 days Phosphorus-32 --- l4.3 days Iron-59 --- 6.6 weeks Polonium-210 --- 20 weeks Cobalt-60 --- 5.26 years |
Tritium (H-3) --- 12.4 years Strontium-90 --- 29.9 years Cesium-137 --- 30.1 years Radium-226 --- l,620 years Carbon-l4 --- 5,730 years Plutonium-239 --- 24000 years Chlorine-36 --- 400,000 years Uranium-235 --- 7l0 million years Uranium-238 --- 4.5 billion years |
RTGs: Power from Radioactivity
On January 20, 2006, the United States launched its “New Horizons” satellite to go all the way to Pluto. It should arrive in 2016. It will send back data and photos about that tiny planet. Where will it get the energy for its transmissions?
Solar power? No. Since Pluto is about 30 times further from the Sun than is the Earth, the solar power is reduced by a factor of 30x30 = 900, to about one watt per square meter. Good solar cells convert only about 1/3 of that to electricity (see Chapter 1). That’s too weak to be used.
Other sources were considered, including batteries and fuel cells. But they cannot provide continuous power for 10 years, and they are heavy. Weight is an important consideration, since only a light satellite could be launched at a high enough speed to reach Pluto in a decade.
For these reasons, NASA chose to use radioactivity to supply the power. The satellite contains 11 kg (24 lb) of plutonium-238 (Pu-238). Its radioactivity creates heat at the rate of about 600 watts/kg, for about 6.6 kW total. A thermoelectric generator (wires consisting of different metals in contact) converts about 7% of this heat into electricity, giving about 460 watts of electric power. The combination is called an “RTG” for Radioisotope Thermoelectric Generator.
NASA used Pu-238, which is 1 neutron lighter than the chain-reaction version Pu-239, because Pu-238 decays with a half-life of 87 years. This rate is fast enough for a large number of nuclei to decay over the 10-year mission, and produces enough high power at low weight. Yet, the rate is low enough that the atoms are only partially used up during the 10-year mission, so the power doesn’t decrease too much over that period.
RTGs have been used for many years. The Voyager spacecraft used an early version of an RTG in 1977 for the same reason as New Horizons: it was to be the first satellite to go beyond the planets into “deep space.”[19]
Some people oppose the use of RTGs because it involves plutonium. They argue that a failed launch could cause the plutonium to fall to Earth and cause environmental damage. For the past few years, we have had no facility to produce Pu-238 in the U.S., and so we have been buying this material from Russia. There are now proposals to create new facilities in the U.S. to make it.
Optional: How do we make Pu-238 for RTGs?
You don’t have to know how Pu-238 is made, but you might find it interesting. It is worth reading through this section quickly, just to get a sense of how they do it. You don’t have to remember the details. In a nuclear reactor (discussed in the next chapter) neutrons are absorbed on U-238 making U-239 which then beta decays into Neptunium-239 (Np-239), and then Pu-239. That’s our source of Pu-239, used in bombs. But some of the Pu-239 absorbs a neutron, becoming Pu-240. That (as we’ll see in the next chapter) pollutes the bomb material. As time goes on, some of the Pu-240 absorbs another neutron and becomes Pu-241. Pu-241 is radioactive, and it emits an electron (beta decay, with a half-life of 14 years) to become Americium-241 (Am-241). The americium decays to make Np-237.
At this point, the Np-237 is separated into a special container and put back into the reactor. In the nuclear reactor, the Np-237 can absorb another neutron to become Np-238. This is radioactive with a half-life of 2 days. It emits an electron and become Pu-238. The container is then removed, and the plutonium separated from any neptunium.
Sound complicated? It is. This is radioactivity high-tech. There are hundreds of other processes that produce radioactive materials for special uses. Many of these are for medicine. The medical technician that uses specialty isotopes usually has no need to know the complex technology that is used to produce them.
Smoke Detectors
The most common smoke detectors have a small radioactive source underneath the cover. It is typically an alpha particle emitter, with alpha particles that only travel about 1 cm in the air before they stop. These alpha particles knock electrons off the air molecules, and that makes the air electrically conductive. This conductivity is measured with a battery. If the air is conductive, then the alarm does not sound.
However, if smoke drifts under the cover, then the electrons tend to stick to the smoke particles. That means they are no longer free to move, and the electric current stops. When the electronics detects that electric current is no longer flowing, it sounds the alarm, usually a piercing screech.
Obviously it is important that the battery works. Smoke alarms measure the battery strength; if it gets weak, the electronics emits short beeps to alert you.[20]
Measuring Age from Radioactivity
If a rock has minerals in it that contain potassium, then it is often possible to tell when the rock was first made. That’s because all potassium on Earth contains about 0.01% of the isotope K-40, and K-40 has a nice property: when it undergoes a radioactive decay, it turns into argon gas. This gas is then unable to escape from the solid rock and it accumulates there. The gas can only escape if the rock is melted.
To see how that can be used to measure the age of a rock, let’s consider a specific example. Suppose we find a rock formed from lava, i.e. it was once liquid. We would like to know when it turned solid, i.e. when it became a rock. We look and see if the rock has potassium in it. If it does, then we know part of that potassium is turning into argon gas. We can see how much argon gas is trapped in the rock, and use that information to determine how long the rock has been a solid. This technique, called potassium-argon dating is enormously useful in geology for measuring the age of rocks and of ancient volcano flows.[21]
Archeologists can use the radioactive isotope of carbon, C-14, to measure the age of fossils. This is called radiocarbon dating. C-14 is produced in the atmosphere by cosmic rays. It is absorbed into plants when they create carbohydrates out of atmospheric carbon (by “breathing” carbon dioxide). We eat the plants, or animals that ate the plants, or animals that ate the animals that ate the plants…and so we get C-14 into our bodies. Since the path from the atmosphere to our bodies happens so fast (typically less than a year) our carbon has nearly the same radioactivity as the atmospheric carbon: 12 decays per minute for each gram of carbon.
When we die, and no longer eat food, the carbon-14 decays and it is not replaced. If an archeologist finds a fossil, and measures that each gram of carbon in it is has only 6 decays per minute of C-14 rather than 12, then he knows that creature died one half-life ago, i.e. 5730 years ago. This method is the primary means for measuring ages in archeology.
Suppose the archeologist measures 3 decays per minute. (Remember: 12 decays/minute indicates an age of 0.) What is the age of the fossil? Careful—this is potentially a trick question. Try it, and then check the footnote[22] for the answer.
After 10 half lives, the radioactivity has been reduced by a factor of (1/2)(1/2)(1/2)(1/2)(1/2)(1/2)(1/2)(1/2)(1/2)(1/2) = 1/210 = 1/1024 = 0.001. So instead of 12 decays per minute, the archeologist will measure only 12 decays per 1024 minutes. Such low rates are very difficult to measure, so C-14 is useful only to ages of about 10 half-lives, i.e. about 57,300 years. Beyond that, the rate is too low.[23]
Puzzle: Why doesn’t the carbon-14 in the atmosphere decay away as it does in our bodies?
Answer: It does, but it is constantly replenished by new cosmic rays. The level in the atmospheric carbon is set by the level at which the decay exactly balances the production. That turns out to be 1 C-14 for every 1012 ordinary carbon atoms. And at that density, 1 g of carbon (which will have 10-12 g of C-14) will have 12 decays every minute.
Radioactive alcohol
Let’s return now to another of the anecdotes that appeared at the beginning of this chapter. In the United States, alcohol for consumption must be made from fruits, grain, or other plants. It is illegal to make it from petroleum. (I don’t know why, but it is the law.) Of course, any alcohol made by the fermentation of plant matter contains recent, radioactive carbon-14. In contrast, petroleum was created by decaying vegetable matter that got buried 300 million years ago. A half-life is only 5730 years, so the petroleum was formed from living matter that died over 50,000 C-14 half lives ago. There is no detectable C-14 left in it. This absence provides an easy way for the U.S. government to test to see if alcohol was produced from petroleum. The United States Bureau of Alcohol, Tobacco, and Firearms tests alcoholic beverages for C-14. If the expected level of radioactivity from C-14 is present, then the beverage is fit for human consumption. If the alcohol is not radioactive, then it is deemed unfit for human consumption.
Environmental Radioactivity
Is all cancer caused by radioactivity in the environment? No. If you live in a typical city, you are exposed to about 0.2 rem of radiation every year. Most of this comes from radon gas seeping up from the rocks in the ground and cosmic radiation coming from space, and some of it comes from medical x-rays, if you get them. In 50 years, the typical American is exposed to a total of about 15 rem of mostly natural radioactivity. To calculate the expected cancer, you follow our rule: take the total number of rem (rem per year multiplied by number of years) and divide by 2500. This gives 15/2500 = 0.004 = 0.4%. But 20% of people die from cancer, not 0.4%, so the cancer must be coming from something else.
What else? Some people think it is food, or pollutants, or something else that we might be able to eliminate. But if we add up all known carcinogens, we still can’t account for the bulk of cancer. So there is some other cause. It could be something as simple as the natural exposure to the highly reactive chemical known as oxygen--which we can’t eliminate unless we give up breathing. Nobody knows.
Volcanic heat and helium balloons
The rock in the Earth is radioactive, largely from potassium, uranium, and thorium in the ground. If you have ever been in a deep mine, you know it is very warm--not cold, as it is in a shallow mine or cave. The reason is that heat is gradually seeping up from inside the earth. Uranium and thorium decays underground and produces a large number of alpha particles. The underground heat is the energy lost by the alpha particles as they slow down in collisions with other atoms. When the alpha particles finally stop, they pick up electrons and turn into helium gas. This gas collects underground along with natural gas (methane), and is extracted along with the methane. It is what we use to fill helium balloons.
The total power generated inside the Earth from radioactivity is about 2x1013 W (watts). That sounds like a lot, but the sunlight hitting the Earth has 2x1017 W, a factor of 10,000 greater.[24] So the energy coming down is much greater than the energy coming up.
The heat generated underground by radioactivity is responsible for volcanoes, thermal springs, and geysers. Under a large glacier (during the ice ages glaciers were several kilometers thick) the heat from the Earth is enough to melt the ice at the bottom of the glacier, and that keeps it slipping.
The Earth is 6370 km thick (to the center), so it is surprising that 20% of the heat comes from radioactivity near the surface, in the “thin” shell of rock known as the crust. The crust is only 30 km thick on average, but it contains a much higher density of the radioactive uranium, thorium, and potassium. At the bottom of the crust, 30 km down, the temperature of the rock is about 1000 C.
Why aren’t most elements radioactive?
This might sound like a silly question, until you learn the answer. We believe that, in the early solar system, most atoms were radioactive. There are intensely radioactive isotopes of hydrogen, oxygen, nitrogen, calcium, and all the other atoms of which we are made. These were once abundant. But most of them had short half lives, from fractions of a second to millions of years, and as a result, most of them decayed away.[25] Now, 4.6 billion years later, we are left only with three kinds of atoms: those that are not radioactive (e.g. C-12), those that have very long half lives (e.g. K-40 and uranium), and those that have been produced in the recent past (e.g. C-14).
Optional: the cause of radioactivity –
the “weak force” and tunneling
Chemical explosives must be triggered, e.g. gunpowder is exploded by the impact of the hammer of the gun, and TNT is usually detonated with the help of an electrical signal. What triggers the nuclear explosion of radioactivity? Alpha decay and fission (to be discussed soon) come from a quantum-mechanical phenomenon called “tunneling.” In tunneling, a particle that is tightly bound to another one by nuclear glue (the gluons) makes a “quantum leap” away, to a location where the force is weak. At this distance, the force on the particle is dominated by its electric charge, and it is strongly repelled. That repulsion is what gives the particle its high energy.
For beta radiation, the answer is totally different. For many years, it was hypothesized that there was a new “force” in the nucleus that was responsible, a force that was so weak that didn’t do anything else except, once in a while, trigger radioactive decay. We now know that this force is real, that it is related to the electric force, and that it operates in such a way that it triggers the explosion. Because of its history, it is called the “weak force.” The fact that the force is weak means it has a very low probability of acting in any one second. For example, it takes 5730 years for a C-14 atom to have a 50% chance of decay.
We now know that the weak force can do more than just cause beta decay. It can also put a force on the particle. The mysterious particle called the “neutrino” has no electric charge. It does not feel electric forces; it only feels the weak force and gravity. Despite its name, the weak force on the neutrino is stronger than the force of gravity. When a neutrino goes through the Earth, there is a small but non-zero chance that it will collide with one of the atoms in the Earth, because it can feel their weak force.
There are particles that don’t feel the weak force at all. The most important one is the particle called the photon. A photon is a particle of light, sometimes called a “wave packet” of light. (We’ll discuss these further in Chapter 10.) X-rays and gamma rays are also photons. Photons don’t feel the weak force, but they do feel gravity. They pick up energy when falling in a gravity field, and their paths are deflected when traveling close to a massive body like the Sun or the Earth. Another particle that we think does not feel the weak force is the graviton, a particle consisting of an oscillating packet of gravitational field.
Is radioactivity contagious?
By this, I mean: if you are exposed to something that is radioactive, do you become more radioactive yourself? Do you “catch” it, like you catch a cold? In the world of science fiction the answer is yes. People exposed to atomic bombs come away glowing in the dark. But in the real world, the answer is no, at least most of the time, for most kinds of radioactivity.
There are two ways that you can become more radioactive by being exposed to radiation. The first is if you actually get some radioactive material stuck on you, or if you breath it in. You don’t really become radioactive, you just become dirty with radioactive dirt. This could happen if radioactive debris from a bomb lands on you, or if you touch radioactive dust while taking a tour of the inner parts of a nuclear reactor. (When I did this recently I had to wear special clothing, and I was instructed to touch nothing.)
But there is a kind of radioactivity that really can make you radioactive: neutrons. Some forms of radioactive explosions emit neutrons, and when these hit your body, they can attach themselves to the nuclei of your atoms. For example, if you add 2 neutrons, you can turn your non-radioactive C-12 nucleus into a radioactive C-14 nucleus. In reality, to do this would require so many neutrons that you would be dead from radiation illness. But objects exposed to intense neutrons do become radioactive.
Forensic radioactivity: “neutron activation”
Radioactivity is unique in its ability to detect a tiny number of atoms hidden amongst a vast number of others. In your body, only one carbon out of 1012, i.e. only one per trillion, is radioactive. Yet we can count the number from the decays, because they emit such energetic particles. Suppose you wanted to detect an atom that is not radioactive? Then the trick is to make it radioactive by hitting it with neutrons. If it makes a unique radioactive isotope, then its presence can be measured. This clever technique is called “neutron activation” and it is a very useful way to detect elements and their isotopes that are present at tiny (part per billion and less) amounts. Such rare constituents can sometimes be used as “fingerprints” to identify the factory in which an object was made, or the part of the world where it came from.
To activate a sample, it is placed in a nuclear reactor, which bombards it with a large number of neutrons. The sample is removed, and its radioactivity is measured to look for the characteristic rays for the desired element.
In 1977, this method was used by Luis Alvarez and his team to search for the rare element iridium. They found enough iridium to conclude that it must have come from an extraterrestrial impact (since meteors, comets, and asteroids contain abundant iridium) From this discovery, they concluded that a large impact had taken place 65 million years ago--at the very time that the dinosaurs went extinct.
The glow of radioactivity
Objects that are intensely radioactive can cause the surrounding air to glow. That’s in part because the radiation can knock electrons off the air molecules, and when those electrons re-attach they emit light. Likewise high-energy electrons can cause water to glow. But such a glow is usually seen only at levels of radiation that are extremely high, such as inside a nuclear reactor.
Even weak radiation can cause strong light emission if it hits special materials called phosphors. In a phosphor, even weak rays have their energy absorbed by the molecules of the phosphor. A short time later the atoms release this energy in the form of ordinary light.
The front of a TV (or computer) screen is made of red, blue, and green phosphors. Take a magnifying glass up to a color TV screen and look at it. You will not see any white phosphors--only red, green, and blue. (These phosphors, despite their name, are not made of the element phosphorus, but of other materials.) When the phosphors are hit by electrons (cathode rays) they emit light, and that is what creates the image. If you put a radioactive material near your TV screen, the rays emitted from it would also make the TV screen glow--provided that they can get through the thick glass.
Radioactive watches and luminous dials
Before the dangers of radioactivity were completely recognized, watches had their dials painted with a combination of phosphor and the radioactive element radium. They glowed brightly in the dark. They also gave people cancer, particularly the workers who painted the dials (and licked their brushes to straighten the fibers). It is not legal to make watches with radium dials anymore, although you can sometimes find one at a flea market.
Not all radioactivity is equally dangerous. A watch with a radium dial emits enough gamma rays that it could double your yearly exposure to radioactivity from natural sources. Many people would conclude that such an exposure is not a big risk, but there is greater risk if the radium leaks from the watch. The craftsmen who painted the radium on the watch dials did get very large doses, and several of them died from effects. In the United States, radium dials are now illegal. I do wear a watch that contains tritium (H-3), the radioactive isotope of hydrogen that has a half-life of 12 years. The tritium emits beta rays, and when these hit the phosphor on the dial of the watch, the phosphor glows. In 12 years, unfortunately, my watch will glow only half as brightly.
The low energy electron (beta particle) emitted when tritium decays has so little energy that it stops within a few thousandths of a centimeter. So it never gets out of the watch. It goes only far enough to make the phosphor emit light.
Some day we may have computer screens illuminated with tritium. Right now it is too expensive; most people mistakenly fear anything that is radioactive, so people haven’t built factories to make tritium cheaply. But with a tritium screen, it would be on all the time, with no battery needed. Of course, after one half-life (about 12 years), the screen would be only half as bright, assuming you still owned the same computer 12 years later.
Optional: if you want to learn more about the risks of radium and tritium watches, look at
www.orau.org/ptp/collection/radioluminescent/radioluminescentinfo.htm
Plutonium
Small particles of radioactive material, such as plutonium, can be very dangerous. Even a tiny dust mote can contain 1014 plutonium atoms. If you were to breathe in a large number of such particles, and they got lodged in your lungs, then one small region of your lungs could receive large doses by having billions of nuclei decay at the same spot. This is why people worry about plutonium. It has been called “the most poisonous substance known to man.” That is incorrect, and a bad exaggeration, but when you hear it said (and you will), you will know that the origin of the fear is the possibility of small particles lodged in your lungs. Plutonium is less toxic than, for example, botulism toxin. Large pieces of plutonium are much less dangerous--unless they are used to make a nuclear bomb.
The author’s mentor Luis Alvarez used to keep a piece of plutonium on his desk, as a paper weight. (That was when he worked at Los Alamos, on the atomic bomb project.) Why didn’t the plutonium cause cancer in his hand? The reason is that the radiation from plutonium consists of alpha particles that slow down rapidly when traveling through matter. They will be stopped by piece of paper. So although the alpha particles enter the skin, they enter only the outer layers of skin, which are either dead already, or soon to be shed. (The primary way that skin protects itself from carcinogens is by constantly shedding its outer layers, and replacing them with fresh skin from underneath.) In contrast, in the lungs the live cells are in contact with the atmosphere. That is also why lungs are so vulnerable to cancer from smoke, whereas the skin is not.
Some people have suggested that terrorists might make a plutonium bomb, not a nuclear explosion, but just one that will take a piece of plutonium and blow it into small particles that could get into the lungs. That is possible, in principle, although getting a metal into particles of the right size is very difficult to do. A terrorist might kill more people if he were to make a botulism-toxin bomb instead of plutonium one. And botulism toxin is much easier to obtain. (It often appears in home-made mayonnaise if the mayonnaise is not kept properly cool.) We’ll discuss the dangers of plutonium in more detail later in this chapter.
Fission
Fission refers to a special kind of radioactivity, the sudden splitting of a nucleus into two or more large parts. It was named in analogy to the fission of biological cells. Fission occurs in two forms: spontaneous and induced. Spontaneous fission is almost non-existent in nature.[26] It does occur in some artificially produced isotopes.
In spontaneous fission, the nucleus behaves like other radioactive nuclei, i.e. it sits around unchanged for typically one half-life, and then at some random time it decays by suddenly flying into pieces.
A second kind of fission is induced fission. This kind is far more important for this book. Induced fission can occur if the right kind of nucleus is hit by a neutron. The neutron is absorbed, and the resulting nucleus, even with just this one tiny addition, becomes unstable, and fissions. This kind of fission is the fundamental basis of both nuclear reactors and nuclear weapons (e.g. the “atomic bomb”). We’ll discuss induced fission in more detail in Chapter 5.
When a nucleus fissions, the bulk of the mass is usually divided into two unequal pieces called (appropriately) fission fragments. These pieces are usually radioactive with relatively short half lives (from seconds to years), and they can be very dangerous to humans. They are the primary source of the residual radioactivity after a nuclear weapon has been exploded, and the main danger in radioactive fallout from such a weapon.
Fusion
Fusion is the source of energy for the Sun, and as a consequence, it is the ultimate source of energy for virtually all life on Earth.[27]
When Charles Darwin published his book The Origin of Species (1859), he had a lot to say that went beyond evolution. He found fossil sea shells high up in the Andes mountain ranges and concluded that these regions had once been under the sea. He estimated that the Earth must be at least 300 million years old, based on his calculation that it would have taken that long for erosion to create some of the great valleys in England. That was adequate time, he concluded, for natural selection to account for changes in species.
But when his book was first published, it received the criticism of the great physicist William Thompson (later knighted as Lord Kelvin and whom the Kelvin temperature scale is named after). He told Darwin that the Earth could not possibly be that old, or the sun would have completely burned out. Even if the Sun were made completely out of coal, it would have used up all its energy long ago. A more energetic source, Kelvin suggested, was meteors! (Even Kelvin knew that meteors carried much more energy than coal.) Kelvin estimated that meteoric heat could account for a solar lifetime as long as 30 million years. But 300 million years was not possible, at least according to all the physics known at their time.
Darwin
had no answer. In the second and later editions of his book, he removed
his thesis that the Earth was hundreds of millions of
years old.[28]
Of
course, we now know that Darwin’s original conclusion was correct. The
Earth is about 4,500 million years old. The Sun has been burning for
longer than that. It isn’t coal that provides the energy, or meteors, but
nuclear reactions. But the source is not radioactivity, or even fission. It is
a kind of nuclear reaction that we have not yet discussed: fusion.
“Fusion”
refers to the coming together of particles, in contrast to “fission” which is the
separation of particles. It may seem strange that you can get energy by
bringing particles together, but it is true, if you pick the particles
correctly. The basic source of fusion in
the Sun comes from bringing together 4 hydrogen nuclei to make helium. In
the process, other particles are also created. In a typical solar fusion,
in addition to the helium we will get 5 gamma rays (for which we use the symbol
g), 2 neutrinos (n), and 2 positrons (e+). (A positron is just like an electron, but
while the electron has a negative electric charge, the positron has a positive
charge.) In symbols, we write solar fusion as:
4 H ą
He + 2 e+ + 2 g + 2 n
You can read this formula as: 4 hydrogens fuse together and then break apart into (that’s the arrow) 1 helium, 2 positrons, 2 gammas, and 2 neutrinos. Most of the energy is in the positrons, gammas, and neutrinos, not in the helium.
Isn’t that fission? Notice that there are more particles after the reaction than before. So why is this called fusion, rather than fission? There is no really good reason for this name. It is really based on the convention that a new element has been created, helium, that is heavier than any of the nuclei that we started with (which were all hydrogen). In fission, the elements created (the fission fragments) weigh less than the original uranium or plutonium.
It
is all the light particles (e+, g,
n) that carry most of the kinetic
energy. The neutrino escapes out of the Sun, but the other particles
collide with other atoms (mostly hydrogen), share their energy with these
atoms, and heat the sun. It is this radioactively-induced heat that makes
the sun shine. The energy released in one fusion reaction is typically 25
MeV.
What is an “MeV”? Let’s
begin with the unit called the “electron volt,” abbreviated “eV” (small e,
capital V[29]).
The eV is useful when talking about individual atoms, since chemical reactions
typically have an energy between 0.1 and 10 eV. An MeV is a million
eV. Here are some conversions that I recommend you need not bother to
memorize: 1 eV = 1.6 x10-19
joules = 3.8 x10-23 Calories.
One “mole” of material has 6 x1023 atoms. In chemical reactions, if
each atom releases 1 eV of energy, then the energy released is 3.8 x10-23
x 6 x1023 = 23 Cal per
mole.
To compare this with previous work: our table in Chapter 1 shows that methane, burned in air, releases about 13 Cal of heat per gram. One mole of methane is 16 g, so methane releases 13 x 16 = 208 Cal per mole. That’s about 9 eV per molecule.
Why the world is interesting
The Sun is a star, and the fusion that takes place in the Sun
is very similar to the fusion that takes place in stars. If the only
fusion that took place was the combining of 4 hydrogen atoms into 1 helium
atom, then the world would be a very dull place. The reason is that
complex life, as we know it, requires heavier atoms such as carbon and
oxygen. Not much of interest (life, intelligence) can happen when all you
have is hydrogen and helium. The only molecules you can get are H2 molecules. Carbon makes very
complicated molecules (such as DNA) and that makes interesting life possible.
We believe that the heavier
atoms such as carbon and oxygen are created inside stars. Carbon is formed
from the fusion of three helium atoms. All the carbon in your body and all
the oxygen in the atmosphere was once buried deep in a star, where it was
created. Fortunately for those of us who like an interesting world, that
star eventually exploded, spewing its debris into space. Eventually, the
material clumped up to form a new star (which we call the Sun) and a bunch of
planets (Earth, Venus, Mars, etc.). With all the carbon and oxygen on the
Earth, life (as we know it) was possible. We are literally made out of the
ashes of an exploded star. The Sun is a secondary star, created from such
debris of an earlier star.
Optional: the details of fusion
You don’t have to know the contents of this section unless
you are interested. The fusion reaction that we gave above, with 4 hydrogens
turning into 1 helium and a bunch of other things, does not usually take place
in just one step. If the star is a primary star, made of only hydrogen and
helium, then the first step is that 2 hydrogens will combine to form a
positron, a neutrino, and a deuteron (a particle with a nucleus containing one
proton and one neutron). In symbols, the reaction looks like this:
H + H
ą d + e+ + n
Even though the formula shows hydrogen as “H,” it normally takes place in such a hot place that its electron has been knocked off, i.e. it is a plasma. Thus the hydrogen in the equation is really just a proton. The electron, moving in the plasma, doesn’t take part in the reaction.
Next, the deuteron combines with a hydrogen atom to create an isotope of helium called “helium-3” and written as 3He:
d + H ą 3He + g
Finally, two helium-3 atoms combine to form ordinary helium:
3He + 3He
ą
He + 2 H + g
Note that in the end, the original hydrogen atoms have been transformed into helium plus a few other things.
Why isn’t the Earth a star?
Why doesn’t fusion take place on the surface of the
Earth? Or inside a tank of hydrogen gas? The simple reason is that
they aren’t hot enough. Why does that matter?
The
nuclei of all the elements have protons and neutrons in them. The protons
give them a positive charge. Positive
charges repel, as we will discuss in more detail in Chapter 6. In ordinary
matter, two nuclei will never get very close because of this repulsion.
To
overcome the repulsion, you have to give the nuclei enough energy that they are
not stopped by the electric force. Energetic atoms are hot atoms. So
if you heat the atoms enough, they will have enough kinetic energy to allow
their nuclei to overcome the electric repulsion and touch. The temperature
that you need depends on the specific fusion reaction, and how often you need
to have the fusion take place. The temperature in the center of the sun is
believed to be about 15 million K. That’s hot, but not too hot. Even at 15
million C what takes place is a relatively slow fusion; most of the hydrogen
fuel has not yet burned. Some stars, which are hotter, completely burn out
in a few million years, and that doesn’t give planets around them enough time
for interesting life to evolve.
How did the Sun get hot enough to ignite fusion?
Of course, the Sun is very hot now, because of the fusion that is taking place. But how did the Sun get hot in the first place? We believe it was simply the gravitational attraction of the matter that made up the Sun. When all that matter got pulled together, the mutual gravity gave it kinetic energy; when it all settled, that energy was converted into heat. This will happen only if the object is big enough so that the temperature rises over a million C. If the mass isn’t that large, then the fusion isn’t ignited and the object never turns into a star. In fact, the definition of a star used by most astronomers is an object that is large enough that the heat at the core ignites fusion.
The outer surface of the sun is not that hot. Its temperature is about 6000 K. That is not hot enough for fusion. All of the fusion takes place at the core of the sun. The heat works its way out, and the surface then glows at a much lower temperature.
Jupiter is not quite big enough to become a star. Its mass is about 0.1% of the Sun’s. It is believed that to become a star (i.e. to ignite fusion), an object must be substantially larger, about 8% of the mass of the sun, almost 100 times more massive than Jupiter.
Fusion for Power
One possible fuel for fusion is hydrogen. There is lots of this in the oceans, since they are made of H2O. Moreover, when you burn hydrogen in fusion, all you get is helium, a harmless gas, useful for balloons and (as we will discuss later) to cool superconductors. It sounds like the ideal energy source, to replace the burning of fossil fuels and nuclear reactors.
Fusion for power has been a goal of scientists ever since the 1950s. Alas, it has proven difficult to achieve. The problem is simple: most schemes to produce fusion require temperatures hotter than in the center of the sun: millions of degrees C. If we are going to use fusion to generate electricity, then we need hotter temperatures so that the fuel will burn quickly. The plans for controlled thermonuclear reactors (CTRs, in the jargon of the energy business) have temperatures of 100 million C. If you want the reactions to take place in a millionth of a second (to make a hydrogen bomb) then the temperature must be even greater.
Anything that hot explodes. That’s the fundamental problem. But there are possible solutions. One of these is called “magnetic confinement” and uses magnetic field to hold the hot hydrogen. Another is to fuse just little bits of hydrogen, and let it explode. Why not? Isn’t that what we do with gasoline and air in our automobiles? That is an approach being developed too. Finally, there are schemes for “cold fusion.” Most of these have been shown not to work, but there are some new ideas that show some promise. I’ll discuss these further in the next chapter, on Nukes.
Optional: Quarks and gluons
There are two types of quarks in these particles: up quarks and down quarks.[30] A proton consists of two up quarks and one down quark. This fact is often written in a form that looks like an equation (since it has an equal sign): p = uud. In this notation, p stands for the proton, u for the up quark, and d for the down quark. But this isn’t really an equation; there are no numbers that you could put in to make the two sides equal.
A neutron consists of two down quarks and one up quark, and that is written in an analogous way: n = udd.
What are quarks and gluons made of? We don’t know, but some people believe they are made of the ultimate elementary particle called a string. That’s what string theory is all about. There is no strong evidence supporting string theory.
What are electrons made of? Not quarks. In fact, an electron appears to be very much like a light quark itself. Just as for quarks, it is possible that electrons are made of strings. We don’t know. Maybe they are made of nothing smaller. Maybe they are really the final and truly elementary particles. We don’t know.
Back to the beginning
Look at the anecdotes that opened this chapter. Do they seem less surprising now? If they were surprising when you first read them, why?
Chapter Review
Radioactivity is the explosion of the atomic nucleus, the small core of the atom that has only 10-5 of the atomic radius and 10-15 of its volume, but over 99% of its mass. The pieces that shoot out from this explosion are called “rays” or “radiation.” Important kinds of radiation include alpha particles (2p2n), beta particles (electrons), and gamma rays (bundles of energetic light).
The nucleus has over 99% of the mass of the atom, but only 10-5 of the radius and 10-15 of the volume. It is made primarily of protons and neutrons, which are in turn made of quarks. The name of the element is related to the number of protons. Different isotopes of the same element have different numbers of neutrons.
When radiation passes through matter, it breaks apart molecules and knocks electrons off atoms, leaving charged particles called ions. The track of ions can be made visible in a cloud chamber, or (if the material is a phosphor) by emitted light.
The path of damage done by radiation can cause radiation sickness, if it is sufficiently intense. The LD50 for this is 300 rem, and each rem would take about 2 billion gamma rays per square centimeter. At lower levels, the main damage is to DNA and the possibility of inducing cancer. The linear hypothesis states that the number of cancers depends only on the rem dose, and not on the number of people who share this dose. In this picture, about 2500 rem cause one cancer. The linear hypothesis has not been tested for low doses. If it is correct, then the Chernobyl nuclear accident will kill about 24,000 people from cancer. Those people will not be easily identified, since about 20% of people die of cancer from other causes.
Most natural material, including your body, is radioactive from K-40, C-14, and other natural materials. This fact can be used to estimate the ages of rocks and bones.
Radioactive decay follows the half-life rule: for every additional half-life (5730 yrs for C-14), another half of the nuclei will decay. Nuclei die in this way but, until they die, they do not age. This is one of the mysterious behaviors that comes from quantum mechanics.
Natural radioactivity in rock is the source of heat within the Earth, including the heat responsible for volcanoes. Alpha particles emitted by K-14, uranium and thorium, slow down and attract electrons, and become helium atoms--used in toy balloons.
Plutonium-238, made in nuclear reactors, produces enough heat from its radioactivity that it is used to provide power for satellite missions to deep space.
We believe that originally, when the sun was formed, most elements were made up of radioactive isotopes. The radioactive ones have now mostly decayed, so we are left primarily with those that are not radioactive.
The two main causes of radioactive decay are tunneling (a kind of quantum leap), and the weak force. The ghost-like particles called neutrinos are sensitive only to the weak force and to gravity. They are so strongly produced by the Sun that over ten billion of them pass through each square centimeter of your body every second.
Most radiation does not make the material it hits radioactive, with the exception of neutrons. The radioactivity that neutrons induce can be used to find trace amounts of rare materials such as iridium (used to understand the dinosaur extinctions).
Plutonium is a man made radioactive element. The main isotope Pu-239 has a half-life of 24,000 years. It is used in nuclear bombs and reactors. It is toxic, but not quite as much as other chemicals including botulism toxin.
Fission refers to the breakup of a nucleus into two or more large parts. Although it happens naturally, the usual method is to induce fission with neutrons. Fission is used in nuclear reactors and in uranium and plutonium bombs. Fusion refers to the coming together of nuclei to make a larger one. Fusion usually produces many small pieces too. Fusion of hydrogen into helium produces the energy that drives the Sun. Most of the heavier elements (including carbon, nitrogen, and oxygen) are the elements that make life “interesting” and were created by fusion within a star. Fusion usually takes high temperatures to get started, but then it produces enough heat to keep the process going.
Discussion Topics
Linear hypothesis. What do you think about the linear hypothesis? If the deaths aren’t observable, should they be counted, even if the linear hypothesis has a good basis in theory?
Hidden deaths.
“If a tree falls in the woods, and
nobody ever observes it (or its consequences), did it happen?” That is a
favorite quandary of philosophy students.
Some people argue that since deaths from very small levels of
radioactivity cannot be detected, statistically, that therefore they might as
well be ignored. Do you agree?
The danger of double beds. Of course, we can’t get away from our own radioactivity. But think about double beds. If we spend a lot of time very close to someone else, we are exposed to their radioactivity as well as our own. Suppose we spend 1/3 of a day in close proximity to another person. Then we might expect to increase our potassium-induced cancers by about 1/3, or about 13 per year. Actually, only their gamma rays will reach you; the beta rays (electrons) will stop in their own bodies. The gamma rays are 10% of the radiation, so the potassium-induced cancers will only be about 1 per year. So if everyone in the U.S. slept in double beds, the number of additional cancers expected in the next 50 years is 50.
That may sound silly. But remember, those are 50 people who otherwise would not die of cancer. And that’s just in the U.S. The current world population is about 6 billon people, 20 times larger than that of the U.S. alone. So over the world, we expect not 50 deaths from double beds, but 1,000.
Do you think we should do something about the double bed crisis? In the 1950s, many married couples slept in twin beds. (See a replay of any 1950s situation comedy for evidence. If they ever show the bedroom, it will have twin beds.)
Should we return to twin beds? We could instead install shields between people that protect them from each other’s gamma rays.
At what point is it silly to worry? What level of “additional cancer” would you consider to be negligible? Would you accept a different level of risk for yourself than you would allow for the entire U.S.? A risk of 0.000006 sounds negligible for me, but 500 preventable cancers in the US sounds substantial--yet they represent the same numbers! This is at the heart of a fundamental paradox for public policy, and I don’t have the answer. (It is also a great opportunity for a demagogue looking for something to use to scare people.)
Lying with statistics. If I wanted to scare you, I quote 13,000 deaths from double beds. If I wanted to make you feel the risk was negligible, I would tell you that the chance of you getting cancer from sleeping in a double bed is 0.000002. (That number is 0.000006 divided by 3.) Both numbers are correct. So be wary when you hear frightening statistics; see if there is a way of stating them that might lead to a different conclusion.
Cancer rates. The cancer rate in Denver is lower than in Berkeley. Some people have argued that this shows radiation protects you from cancer. Is this valid? Why not?
Public perception. Think about a person who lived so near the Chernobyl accident that he received a dose of 100 rem, and then contracted cancer. A rem does of 100 gives a 1/25 = 4% chance of cancer. Therefore his chance of getting cancer has been increased from 20% to 24%. But what is the public perception? Will the friends and relatives believe that the cancer was induced by the accident, even though only 1 out of 6 (i.e. 4 out of 24) of such people actually had their cancer triggered by the radioactivity rather than by the usual causes?
Radiation and Cancer. Khidhir Hamza was the chief Iraqi bomb designer, before he defected to the U.S. He wrote a book about his experience, called Saddam’s Bombmaker In it, he says:
The Russian reactor was also targeted. Somebody must have closely studied its layout, because the reactor was destroyed, though not the fuel in the core. (Later, Saddam would claim to UN inspectors that it was destroyed.) The next day, stupidly, tragically, senior scientists and engineers were called in to salvage the computers and electronic equipment, and found themselves wading through radioactive water. Many of them, including Basil al-Qaisy, one of the best electrical engineers in Iraq, would eventually come down with cancers.
from Khidhir Hamza and Jeff Stein, Saddam’s Bombmaker (New York: Scribner, 2000), p. 246
Discuss this paragraph in view of what you know. Did Saddam’s chief bombmaker make a mistake?
Ethics of the neutron bomb. Suppose we had developed the neutron bomb in World War II, instead of the ordinary atomic bomb. The neutron bomb kills people, but does much less damage to buildings. But also suppose that years later, the military invented a bomb that did both--the “atomic bomb.” And President Carter decided that the atomic bomb, because it did more damage, would be useful to deter war in Europe. Would the public have decided that that Carter did the right thing, that replacing the neutron bomb with one that did more destruction, was more humane? Some people would argue that this conclusion is the logical consequence of the criticism of the neutron bomb. What do you think?
