2. Atoms and Heat
Quandaries
When the asteroid hit the Earth 65 million years ago, it had a kinetic energy equivalent of 100 times its own weight in TNT. In the impact, virtually all that energy was turned into heat. The temperature of the rock (turned into vapor) was over a million Celsius, over a hundred times hotter than the surface of the sun.
Why? How does kinetic energy turn into heat? What is heat? How did this lead to an explosion?
All objects sitting in a room should reach the same temperature. Yet if you pick up a cup made of glass, it feels cooler than a cup made of plastic. Many people unconsciously recognize plastic by its relative “warm feel.”
How can two objects be the same temperature and yet one feels cooler? What mistaken assumption are we making?
Many scientists are worried that the Earth is warming. Some models predict that the continued dumping of carbon dioxide into the atmosphere (from the burning of fossil fuels) could warm the Earth by about 2.5 C, equal to 4.5 F. If this happens, we expect the oceans to rise by several feet--even if no ice melts. Many coastal cities, particularly those in Florida, would go underwater.
Why should the sea level rise if no ice melts?
When we heat our homes by burning fuel, we are wasting energy. We could do a much better job by pumping heat from the cold outdoors into our homes.
Pump heat from the cold outdoors? This sounds like nonsense. Isn’t the burning of fuel 100% efficient, since all the energy goes into heat? How could we possibly do better than that?
Atoms and Molecules and the Meaning of Heat
Press your hands together hard and rub them vigorously for about 15 seconds. (It is actually a good idea to do this right now, before you read further, if nobody is watching.) Your hands feel warmer. The temperature of the skin has risen. You turned kinetic energy (energy of motion) into heat.
In fact, heat is kinetic energy, the kinetic energy of molecules.[1] Your hands feel warmer because, after rubbing, the molecules are shaking back and forth faster than they were prior to your rubbing. That’s what heat really is: the shaking of atoms and molecules, rapid in speed, but microscopic in distance.
This is a good time to discuss the makeup of matter. All substances are made of atoms, and there are only about[2] 92 different kinds of these: hydrogen, oxygen, carbon, iron, etc. A complete list appears in a chart known as a periodic table, shown below:
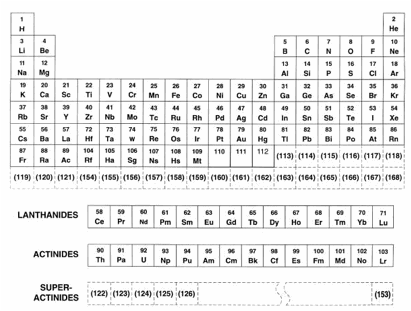
Each of the atoms in the periodic table has a number associated with it called the atomic number. It represents the number of protons in the atom; it is also the number of electrons in the atom (usually). The atomic number of hydrogen is 1, for helium the atomic number is 2, for carbon it’s 6, for oxygen it’s 8, and for uranium it’s 92.
Molecules are combinations of atoms that stay clumped together. A molecule of water is written “H2O,” meaning it is made of two atoms of hydrogen (that’s the H2) and one atom of oxygen (that’s the O). Helium molecules contain only one atom (He), and hydrogen gas molecules contain only two attached atoms of hydrogen (H2). But molecules can be very large. The molecule known as DNA, which carries our genetic information, can contain billions of atoms.[3] When molecules break apart or come together it’s called a chemical reaction.
In all materials, the molecules are constantly shaking. The more vigorously they shake, the hotter the material is. When you rub your hands together, you make the molecules in your hands shake faster. How fast do they shake? The answer is startling: the typical velocity of shaking is about the same as the speed of sound, about 700 mi/h, 1000 ft/s, or 330 m/s. That’s fast. Yet the particles (at least in a solid) can’t travel very far. They bump into their neighbors and bounce back. They move fast but, like a runner on a circular track, their average position doesn’t change.
Atoms are too small to be observed with an ordinary microscope. Their size is about 2x10-8 cm = 2x10-4 microns[4]. If you move across the diameter of a human hair (typically 25 microns), you will encounter 125,000 atoms from one side to the other. A red blood cell (8 microns across) has about 40,000 atoms spanning its diameter. Some molecules are so large (such as DNA) that they can be seen under a microscope, although the individual atoms in the molecules can’t be resolved.
Even though you can’t see atoms, you can see the effect that their shaking has on small, visible particles. With a microscope, you can see the shaking of tiny bits of floating dust (1 micron in diameter). This phenomenon is known as Brownian motion.[5] The shaking comes from the dust being hit on all sides by air molecules, and if the dust is sufficiently small, this bombardment does not average out. For a wonderful simulation of this effect, see the Web site at
http://ephysics.physics.ucla.edu/ntnujava/gas2D/ebrownian_motion.htm
There is a similar site at
www.phys.virginia.edu/classes/109N/more_stuff/Applets/brownian/brownian.html
The Speed of Sound and the Speed of Light
Is it a coincidence that the speed of molecules is approximately the speed of sound? No-- sound travels through air by molecules bumping into each other. So the speed of sound is determined by the speed of molecular motion. Sound traveling through a gas cannot move faster than the velocity of the gas molecules.[6]
You can easily measure the speed of sound yourself. One way is to watch someone hit a golf ball, chop wood, or hit a baseball. Notice that you see the event before you hear the noise. That’s because the light gets to you very quickly, and then you have to wait for the sound. Estimate your distance to the person, and estimate how long it takes for the sound to reach you. If the distance is 1000 feet, then the delay should be about 1 second. (If you do this at a baseball game, then it is helpful to sit as far from home plate as possible.) The velocity is the distance divided by the time.
When I was a child, and afraid of thunder and lightning, my parents taught me a way to tell how far away the sound and light was coming from. For every 5 seconds between the lightning flash and the thunder, they said, the lightning was 1 mile away. If there was a 10-second delay, then the lightning strike was 2 miles away. To me at that age, a mile was about the same as infinity, and so that put me at ease. The rule works because light travels so quickly that it covers a mile in a tiny fraction of a second. In other words, the light arrives virtually instantly. But thunder, since it is a sound, travels at the slower speed of sound: 330 m/s = 1000 ft/s = 1 mi/5 s (approximately) = 700 miles per hour (mph).
Knowing the speed of sound can be useful to measure distances. In 2003, I found myself on a boat some distance from a glacier that was dropping huge pieces of ice into the water. I measured that the sound took 12.5 seconds to reach me. That way I knew that the edge of the glacier was 2.5 miles away (1 mile for every 5 seconds). Until I did this, I had thought I was much closer; the huge size of the glacier had misled me.
The speed of light is much greater: 186,000 mi/s, or 3 x108 m/s. Although that sounds super fast, we can express it in a way that makes it sound much slower. Modern computers take about 1 billionth of a second (a nanosecond) to do a calculation. (Some go much faster, but you should know that a nanosecond is typical.) In that billionth of a second, light travels only about 1 ft (30 cm). That’s why computers must be small. Computers must often retrieve information to do a calculation, and if the information is too far away, it has to waste several cycles to get it.[7] If the computer speed is 3 GHz, then the light goes only 4 inches in one cycle.
Remember:
the speed of light is about 1 foot in 1 computer cycle (1 ns)
The Enormous Energy in Heat
The average speed of the molecules that compose this book is the speed of sound, but they are all moving in random directions. Suppose I made them all move in the same direction. Then the entire book would be moving at the speed of sound, 720 miles per hour. Yet the total energy would be exactly the same.
This example illustrates the enormous energy that is contained in the heat of ordinary objects. Unfortunately, it is often not possible to extract that energy for useful work. We’ll discuss this further when we get to the section on heat engines. There is no good way to change the directions of the shaking so that all the molecules move together. Yet we can do the opposite. When an asteroid hit the Earth 65 million years ago, all the molecules were initially moving at 30 km/s in the same direction. After the impact, the directions were all different.
When kinetic energy is turned into heat, we can think of this process as coherent, regular motion becoming randomized. The molecular energy changes from being neatly “ordered” (all molecules moving in the same direction) to being “disordered.” The term disorder is very popular in physics. The amount of disorder can be quantified, and that value is given the name entropy. When an object is heated, its entropy (the randomness of its molecular motion) increases. I’ll discuss entropy further at the end of this chapter.
Hiss and Snow: Electronic Noise
Radios, when tuned in between stations, sometimes give a hissing sound. What is the origin of that hiss? Old TV sets, when there is no station present, show white spots jumping on the screen that reminded people of snow. What is the snow?
The surprising answer is that the snow and the hiss are due to the same thing: electrons jumping around in the electronics of your set. They are in constant motion due to heat, and when there is no other signal present, you get to watch (or listen) to them move. Even though they are not molecules, they share the energy of shaking.
Lowering the temperature can reduce such noise, and high-sensitivity electronics often have to be cooled to reduce the hiss and the snow. In the Chapter 9 “Invisible Light,” I’ll talk about a device for seeing in very low light that had such a cooling system attached. But too much cooling can cause the device to cease operation, since a transistor (discussed in Chapter 11 “Quantum Physics”) actually depends on the fact that room-temperature electrons have some kinetic energy. Without that kinetic energy, the electrons become trapped and electricity doesn’t flow. If you cool a transistor, and remove that energy, the transistor no longer functions.
Now that we have described heat as the kinetic energy of the molecules (and sometimes of the electrons too), we can address a trickier question: what is temperature?
Temperature
Temperature is closely related to heat. Stop for a moment and think about it. When it is 100 F outside, it is hot. When it is below 32 F, water freezes. But it is very tricky to state exactly what temperature is. It is what you read with thermometers. But what does it measure? The answer is surprisingly simple:
Temperature is a measure of the hidden kinetic energy of the molecules.
By the “hidden kinetic energy” I mean the usually unobserved energy of fast (speed of sound) but microscopic (in distance moved) shaking. When we get to the section on temperature scales, I’ll give the equation that allows you to calculate the kinetic energy from the temperature.
The temperature increases when the average shaking energy of its molecules is greater. (We use the word average because at any given instant, some of the molecules may be moving faster than others, and some slower, just like dancers on a dance floor.) If two objects have the same temperature, then their molecules have the same kinetic energy of vibration.
Here is a surprising consequence of what I just said. Suppose two bars, one made of iron and the other of copper, have the same temperature. Then their molecules must have the same kinetic energy, on average. Will the iron molecules and the copper molecules have the same average speed? The surprising answer is no. The iron molecules will be shaking faster, on average.
In Chapter 1, I said that kinetic energy is given by KE = ˝ mv2. Copper and iron have different masses m. So the heavier copper molecule must have a smaller velocity v in order to have the same kinetic energy KE. See why temperature was once even more of a mystery than heat?
Remember this:
At the same temperature,
lighter molecules
move faster (on average) than
heavier ones.
The Zeroth Law of Thermodynamics
The key discovery that makes temperature a really useful idea is the simple fact that two things that touch each other tend to reach the same temperature. That is why a thermometer gives you the temperature of the air--because it is in contact with the air, so it gets to the same temperature. The fact that objects in contact tend to reach the same temperature was such an important observation that it was given a fancy name: the zeroth law of thermodynamics.[8]
Touch a hot iron object to a cold copper one. Because they are touching, the fast molecules in iron now bang into the slower ones in the copper. The iron molecules lose energy and the copper ones gain. The temperature of the iron will drop and that of the copper will rise. Only when the temperatures are the same does the transfer of energy stop. The “flow” of heat is actually the sharing of kinetic energy. Heat (kinetic energy) is given up by the hot material to the cold one. The flow stops only when both materials have the same temperature.
This means that if you put a bunch of things in the same room and wait, that eventually they will all reach the same temperature. Of course, that doesn’t work if one of the objects is a source of energy, such as a burning log. But if no energy is going in or out of the room, all objects will eventually reach the same temperature.
Where is our hydrogen?
The element hydrogen is, by far, the most abundant element in the Universe. Hydrogen atoms make up 90% of the atoms in the Sun. The same is true for the large planets of Jupiter and Saturn. Yet in the atmosphere of the Earth, hydrogen gas is virtually absent. Why? Where is our hydrogen?
There is a remarkably simple answer, and it comes from the zeroth law of thermodynamics. The Earth once had lots of hydrogen, but we lost it to space. Hydrogen in the atmosphere of the Earth would have the same temperature as the nitrogen and oxygen. Therefore, the molecules of hydrogen have the same kinetic energy, on average. But since hydrogen is the lightest element (its atomic weight is only 1/16 that of oxygen), it must have a higher velocity. Since energy depends on the square of the velocity, the velocity must be a factor of 4 larger (so the square is 16). This high average velocity turns out to be enough for the hydrogen to escape from the Earth like a rocket![9] The Sun and Jupiter have much stronger gravity than the Earth, so they kept their hydrogen. We’ll discuss escape velocity in more detail in Chapter 3 “Force and Gravity.” The Earth lost its hydrogen gas because our gravity is too weak.
The cold death
Stars are very hot, and molecules in space are very cold. Eventually the stars will stop burning, and eventually everything in the Universe may reach the same temperature. By keeping track of everything, we can calculate what that temperature is. If we ignore the expansion of the Universe (see Chapter 12) then the average temperature of the Universe turns out to be –270 C.[10] Because the Universe is expanding, the eventual temperature may be even lower. Philosophers have called this the “cold death” of the Universe, and the thought of it gets some people depressed. But being cold doesn’t necessarily mean life will be uninteresting. A detailed analysis made by physicist Freeman Dyson showed that even as the Universe gets very cold, life can continue, and the complexity of organized thoughts could get greater and greater. That might take additional evolution, but we have hundreds of billions of years for that.
What would life be like in such a universe? What would the descendants of humans look like? Some people estimate that, because of the extreme cold, in order to remain complex, active creatures, they would have to be very large, perhaps as large as planets are now; maybe even bigger.
Temperature Scales
The concept of temperature was invented long before it was understood. It was measured using devices called thermometers. People could make thermometers that would always agree, more or less because (as stated in the zeroth law) it doesn’t matter what material the thermometer is made out of. So temperature became a standard idea. We’ll talk about how thermometers work later in the chapter.
There are two common temperature scales, the Fahrenheit Scale, and the Centigrade scale. Centigrade has recently been renamed “Celsius.”[11] Celsius is also abbreviated C, just like Centigrade, and Fahrenheit is abbreviated F. The scales are defined such that the freezing point of water is 32 F and 0 C, and the boiling point of water is 212 F and 100 C.[12]
We can convert between Fahrenheit and Celsius by the following rules. Let TC be the temperature expressed in the Celsius scale, and TF be the temperature in the Fahrenheit scale. Then
TC = (TF –32)(5/9)
TF = (9/5)TC + 32
Examples (try the equations yourself):
Freezing of water: TF = 32 gives TC = 0
Boiling of water: TC = 100 gives TF = 212
“room temperature” TC = 20 gives TF = 68
Degrees
Until recently, it was common to refer to temperature in degrees. A temperature of TF = 65 was read as “65 degrees Fahrenheit” and written 65oF. However, the word degree doesn’t add any meaning, and some people were confused by it. (It has nothing to do with angles, which are also measured in degrees.) So scientists are now adopting a new convention of dropping the degree symbol. Thus 32oF is usually shortened to 32F. You’ll see it both ways. There is no physics in this; it is just notation. I’ll sometimes use the traditional terminology, just because of the fact that that is how you will hear it used most, and because it sometimes makes it clear that we are talking about temperature.
Note that Celsius degrees are bigger than Fahrenheit degrees. A change of 1C is a change of 9/5 F = 1.8 F ≈ 2F. As an approximation for changes in temperature, remember:
1oC ≈ 2oF
Digression: Which is metric, C or F? The original Fahrenheit scale was designed to make 0 F the coldest temperature that could easily be reached in a laboratory. That was done by mixing ice and salt, and that is what is called 0 F. The temperature of 100 F was originally chosen to be body temperature. (They made a slight mistake, and average body temperature is actually about 98.6 F.) On this scale, water freezes at 32 F and boils at 212 F. When the centigrade scale was officially adopted (by the French, under Napoleon) they decided that the two standard points should be the freezing and boiling points of water. So on the Centigrade scale, water freezes at 0 C and boils at 100 C. Some people think the Centigrade scale was more “metric” than the Fahrenheit scale, and that is nonsense. Both scales were based on standard points 100 degrees apart; they just chose different standard points.
Absolute Zero
What happens if the molecules actually come to a stop, and have zero kinetic energy? When all motion of the molecules stop, we say the temperature of the material is at “absolute zero”. This happens at -273 C = -459 F.[13]
Using this fact, we can define a new temperature scale called the “Absolute” or “Kelvin” scale (named after William Thompson[14]). Physicists find the Kelvin scale to be very convenient because it simplifies equations. For example, if we use the Kelvin scale, then the average kinetic energy E per molecule is given by a very simple equation:
E = 2 x10–23 TK
where TK is the temperature in Kelvin (or degrees Kelvin). The constant, given in the equation as 2 x10–23, is very small only because atoms are so small. Don’t bother learning this number. It is not important to know the numerical value for the kinetic energy of the particles. It is important to know their velocity (1000 ft/s, about the speed of sound) and that if you double the temperature (on the Kelvin scale) then you double the kinetic energy.
The most remarkable fact about this equation is that it doesn’t depend on the kind of material. That’s just the zeroth law again. I find that to be an amazing and surprisingly simple law of physics. Ponder it for a few moments. Temperature is just the hidden kinetic energy. At room temperature, the kinetic energy of the atoms in the air is identical to the kinetic energy of the atoms in this book. That fact eluded scientists for hundreds of years. The only really tricky part is that the energy must be measured per molecule. This equation begins to illustrate what physicists sometimes refer to the “beauty” of physics. It isn’t really beauty in the traditional sense. It is just an insight, a simplicity that is missed by people who don’t study physics.
You can convert from the Kelvin scale to the Celsius scale by subtracting 273:
TC = TK – 273
Thus, for example, TK
= 273 is the same as TC =
0. Put another way, 273 K = 0 C.
The Columbia space shuttle tragedy
On February 1, 2003, the Columbia space shuttle broke apart in flames as it reentered the atmosphere, killing all seven astronauts on board.
The space shuttle always generates enormous heat when it reenters the thicker parts of the Earth’s atmosphere. That’s because it has very large kinetic energy, and to slow down (so it can land) it must get rid of that energy.
To calculate the energy per gram, we need to know the velocity. When the space shuttle orbits, it travels the Earth’s circumference of 24000 mi in 1.5 hours, so its velocity is 24000/1.5 mi/h = 16000 mi/h = 7000 m/s = 22 times the speed of sound. At the time that it began to fall apart, the Shuttle had slowed to 18.3 times the speed of sound. That is known as “Mach 18.3.” We’ll show why it has to move so fast in Chapter 3.
In the optional calculation below, we show that if the kinetic energy of the space shuttle were all turned to heating it, its temperature would rise to
The Mach Rule:
T = 300 M2
where M is the Mach number. This is a useful equation that you will not find in any other textbook. For M = 18.3, this gives T = 100,000 K. That is 17 times as hot as the surface of the Sun. This is why the pieces of the Shuttle glowed so brightly, friction with the air made them very, very hot.
There is no way to avoid this turning of kinetic energy to heat on reentry.[15] The Space Shuttle is designed to have heat-resistant ceramic “tiles” on the bottom surface. During reentry, these tiles face the onrushing air, and glow with a temperature of thousands of degrees. They can lose this heat by conduction with the air and by radiation. They cool off by the time that the shuttle lands.
The shuttle contains little fuel and no explosives. It was the kinetic energy of motion, turned into heat, which destroyed the vehicle.
High Temperatures. Here’s a little trick you might find helpful. Suppose an object (such as a meteor, or the interior of the sun) has a temperature of 100,000 C. How hot is it in K? The answer is 100,273 K. That looks pretty close to 100,000. They differ by only 0.27%. Here is a useful rule: when temperatures are really high, then the temperature in C is approximately the temperature in K.
Optional calculation:
Let’s derive the Mach number equation. Now here is a trick that can allow you to get the answer very quickly. We know that at room temperature (300 K) the molecules in the Shuttle are moving at about the speed of sound, i.e. at Mach 1. Suppose all the kinetic energy of the orbiting shuttle was randomized, i.e. turned into heat. Then the molecules would be moving at Mach 18.3 (since that is how fast the Shuttle was moving). So as the energy of orbit turns into the energy of heat, the molecules hidden motion speeds up by a factor of 18.3.
What does that do to their hidden kinetic energy, i.e. to the temperature? Remember that the kinetic energy is E = (1/2) m v2. So if you increase v by a factor of 18.3, you increase the kinetic energy by a factor of (18.3)2 = 335. That means you increase the temperature by a factor of 335, from 300 K to 335 x 300 K = 100,000 K.
Put another way, if you move at Mach number M = 18.3, and turn your kinetic energy into heat, your temperature will rise to a temperature T = 300 M2. This equation can be used for any Mach number M, and it gives the temperature in Kelvins.
Thermal expansion:
sidewalk cracks, highway gaps, New Orleans levees, and shattering glass
When the atoms in a solid heat up (i.e. they move faster, i.e. their velocity increases, i.e. they get more kinetic energy) they tend to push their neighbor atoms further away. The effect is small, but important: most solids expand a little bit when heated. A typical number, worth remembering, is that a 1 C temperature rise makes many substances expand by somewhere between 1 part in 1000 and 1 part in 100,000.
These sound like small numbers, but the span of the Verrazano-Narrows Bridge in New York City is 4260 ft. When the temperature changes from 20 F to 92 F (a typical seasonal change in New York City), then the length of the bridge changes by about 2 ft.[16]
Another effect of change in temperature is the change in shape of the bridge. Because the suspension cables get shorter in the winter cold, the height of the middle of the suspension is 12 ft higher in winter than in summer. Why is this more than the 2 ft we calculated for the span? The answer is in the geometry: the cables shorten by only 2 ft, but because of the shallow way in which they hang, that makes the center rise 12 ft. Try this with a horizontal string. If you hold it tight, it is straight. But loosen it just a bit, just a centimeter, and it will sag a lot more than a centimeter.
Sidewalk cement is typically laid with grooves between squares about 5 ft = 60 in on a side. In a 1 C temperature change, its length would change by 35 parts in a million, i.e. by 60 inches x 35E-6 = 0.002 inches. For a 40 C change, that is 0.08 in, almost a tenth of an inch. That may not sound like very much, but if there were no grooves, the concrete would be compressed and might buckle, causing random cracks. (Just as with the bridge, and the string, a small expansion can cause a big buckling.) The small groove, placed by the person who paved the cement, gives room to expand and prevents the cracking. (Or, rather, it puts neat cracks in ahead of time, instead of letting ugly, random cracks form.)
Large pieces of cement or concrete will always crack if they are exposed to changes in temperature, unless those cracks are introduced. That creates serious design and engineering problems. Imagine, for example, that you were asked to build levees to protect the city of New Orleans from flood. (Much of the city is below sea level.) You can’t surround it with solid concrete levees, because those would crack when the temperature changed. You would have to make the levees out of individual pieces, with spaces in between. Those would have to be filled with sliding joints or some other flexible material. But if not done properly, those connecting regions might turn out to be the weakest part of the levee.
In fact, that is exactly what happened. In the photo on the next page shows part of the levee in New Orleans that failed after Hurricane Katrina. The levee is clearly made out of rectangular segments; that was done to leave room for expansion. But it was the expansion joints that failed, not the concrete itself. The expansion joints didn’t break from heat – that’s what they were designed for. But they were weaker than the reinforced concrete, so when the pressure of the flood put a great force on the levee, that’s where they broke – at the weakest points.

Levees in New Orleans, broken at their thermal
expansion joints. They didn’t
break from heat, but from the pressure of the flood.
(US
Army Corps of Engineers photo)
Shattering glass
If you heat a glass pan in the oven, then put it in cool water, it will often crack or even shatter. A few decades ago, a special glass was developed that didn’t crack; it was trademarked as “Pyrex,” and is very popular for kitchen glass, e.g. measuring cups and pans. What makes Pyrex special? Why do sudden temperature changes cause some materials to crack and not others?
The glass cracks because the outside cools more rapidly than the inside, making it a different size. It starts to bend, like a bimetallic strip, but glass is brittle, and it breaks. Pyrex is a special glass that expands much less than ordinary glass; that is why it usually doesn’t break when cooled.
Why doesn’t the glass crack when initially heated in the oven? The answer is that when heated slowly, the heat passes through the glass, and all of the glass is at approximately the same temperature. It is the difference in temperature between the inside and the outside of the gas that causes the different expansion, and leads to the cracking.
Tight lids
Tight lids on jars are such a common problem that I own several special devices to help open them, mostly large wrenches that get a good grip on the lid. But my mom taught me a different way to do it: put the lid under hot water for a few seconds. The expansion of the lid, although it is tiny, is often enough to loosen the lid so it can be opened. (I’d use a rag to hold the hot lid.) This works only if the metal expands more than the glass. That happens if the expansion coefficient is greater, or if the lid gets hotter than does the glass.
Global warming and the rise of sea level
Many climate experts believe that the temperature of the Earth is rising because of the carbon dioxide being dumped into the atmosphere by the burning of fossil fuel. (We’ll discuss this in detail in Chapter 10.) The predicted warming over the next 30 years is between 1.5 and 5 C, depending on which model turns out to be more accurate. For the moment, assume that we will warm by 5 C = 9 F.
One of the most surprising effects of the warming is the rise of sea level--not because ice melts (although that does contribute) but simply because water expands so much. The volume expansion of water is 2 x10-4 per degree C. For 2.5 degrees C, that amounts to 2.5 x 2 x10-4 = 5 x10-4 = 0.0005. The average ocean depth is about 12,000 ft. When the oceans expand, they will rise by 0.0005 of this, i.e. by about 6 ft. That would flood much of the coastal areas of the world, including much of Bangladesh and virtually all of the populated area of Florida.[17]
This scenario is scary enough that people have become seriously interested in doing the calculation carefully. More detailed calculations have been done that take into account the expected temperature distribution (global warming is expected to be greatest near the poles, and least near the equator) and the variability of the expansion of water. (When the temperature of water is just above 4 C, it hardly expands at all when heated, and below 4C, it shrinks when heated. Much of the deep ocean is close to 4 C). The 1996 report of the Intergovernmental Panel on Climate Change estimates that, taking all these things into account plus melting of glaciers, the effect would be a rise in sea level between 15 and 95 cm, i.e. between 6 in and 3 ft—enough to cover most of the populated area of Florida.
Thermometers
 Most thermometers make use of the small expansion in order to measure
temperature. They typically fill a small glass bulb with fluid, and attach a
tube with a tiny long hole. When the temperature is raised, the fluid expands,
and moves up the tube. Markings on the tube indicate the temperature.
Most thermometers make use of the small expansion in order to measure
temperature. They typically fill a small glass bulb with fluid, and attach a
tube with a tiny long hole. When the temperature is raised, the fluid expands,
and moves up the tube. Markings on the tube indicate the temperature.
In a real thermometer, the diameter of the bulb (holding most of the liquid) is much greater than the diameter of the tube. Note that the thermometer would not work if both the glass and the fluid expanded the same amount. Thermometers take advantage of the fact that fluids can be found (e.g. mercury and alcohol) that expand more than glass. Alcohol, died a red color, is commonly used because its expansion is particularly large. Most of the alcohol is in the bulb at the bottom. When it expands, it forces fluid into the tube. Without the bulb, the expansion would not be enough to be visible. A large amount of fluid (in the bulb) expands; it has nowhere to go (because the glass holding it does not expand) except up the tube. The tube usually has a vacuum in it so that air pressure does not impede the flow.
Temperature in the shade vs. in the Sun
Why do meteorologists measure temperature in the shade, rather than in the Sun? Aren’t people more interested in the temperature in the Sun! Why don’t they report it?
It turns out that there is a good reason. Thermometers are supposed to measure air temperature. When you place them in a room, they eventually reach the same temperature as the air; that’s the zeroth law of thermodynamics. However, if you put a thermometer in direct sunlight, the red-colored alcohol absorbs more sunlight than does the transparent air. That makes the thermometer hotter than the air. Of course, heat will flow from the thermometer into the air, but if the sun keeps shining on the thermometer, the thermometer will always be hotter. So a thermometer in the Sun does not measure the air temperature. On the other hand, the temperature of the air in the shade is usually the same as that in the Sun.[18] So if you really want to know the temperature of the Sun-lit air, measure it in the shade.
What happens if another object sits in the Sun? It too can get hotter than the air. You’ve probably had the experience of walking on hot sand, or of touching a car that has been sitting out in the Sun. Because these objects absorb sunlight readily, they are often hotter than the air. It is an old tradition in New York City (where I grew up) of publishing newspaper photos on a hot day showing someone frying eggs on an automobile hood. The hood is hotter than the air. It is much hotter. That’s because the Sun is shining on it.
As a consequence, the “temperature in the sun” is not a well-defined concept. Different objects will have different temperatures. Air close to a hot auto will be hotter than air close to a mound of snow, even if they are only a few feet apart. In fact, even the temperature of an object in the sun is not well-defined, since its surface (exposed to sunlight) will usually be hotter than its interior.
Another type of thermometer works on the principle that different metals will expand by different amount. If you take two bands of different metals and bind them to each other, you get a “bimetallic strip.” As one side expands more than the other, the strip bends. The amount of bending will be very large for even a small amount of expansion. The bending metal can pull a lever that moves an indicator over a temperature scale. Thermometers using bimetallic strips are used in oven thermometers and in old thermostats.
Yet a third type, called a digital thermometer (often used in medicine) takes advantage of the fact that the electrical properties of certain materials change when the temperature changes. A small circuit with a battery can measure these changes, and put the result on a digital display.
Does everything contract when cooled?
No. Cold water (below 4 C ≈ 39 F but not frozen) expands when it is further cooled. As it freezes into ice, water expands even more. This is a strange behavior, and it happens because water molecules start arranging themselves into mini structures, even while in a liquid state.
Without this peculiar behavior of water, life on Earth might not have endured. In oceans and lakes, once the water gets colder than 4 C, the freezing water expands, and with its low density it floats on top of the other water. When it freezes, it expands even more, and so ice forms on the surface. This ice and layer of cold water insulates the water below, and keeps it from getting colder.
If cold water were denser than warm water, then in winter the cold surface water would sink to the bottom, and the warm water would rise to the top, where it would be chilled by contact with the cold air. If water contracted when it froze into ice, then even the ice would sink to the bottom. Some people speculate that the entire ocean would eventually reach the freezing point, and turn into ice, and whatever life there was in the water would be killed.
SR-71 spy planes
SR-71 planes flew so fast, that friction from the air heated the outer surface to over a thousand degrees C. The thermal expansion was so great, that if the wings were made in the usual way, they would crack. According to the designers (see the book The Skunk Works by Ben Rich), they solved this problem by making the fittings of the plane loose--almost like the cracks placed in concrete. A good tight fit was obtained only when the metal expanded, at high speed. A tricky consequence of this was the fact that the planes leaked fuel (through these loose fittings) until the outside heated up sufficiently. (I know, this is hard to believe, but it’s true.) An image of the SR-71 is shown below.

SR-71
(NASA
photo)
Conduction
When two objects come in contact, the touching (collisions of surface molecules) allows them to share kinetic energy. The zeroth law implies that the hotter object (greater kinetic energy per molecule) will lose some of its kinetic energy and the cooler object will gain some. Eventually they will be at the same temperature. But this doesn’t happen instantly. Moreover, the rate is different for different materials. We say that different materials “conduct heat” at different rates.
Let’s look at one of the “quandaries” at the beginning of the chapter. Even though both are at room temperature, a plastic cup and one made of glass feel different. The glass one seems cooler. (If you’ve never noticed this, find two such cups now, and do the experiment.) But why should that be? If both objects were sitting together in the room, they were at the same temperature, right?
Yes, the plastic and the glass were at the same temperature. But plastic and glass conduct heat at different rates. Your finger is warmer than room temperature, because you are generating heat in your body at an average rate of about 100 watts. When you touch the glass, it conducts the heat away rapidly, and so the temperature of your fingertip drops slightly. That is what your nerves sense: not the temperature of the glass, but the temperature of your skin. When you touch plastic, the heat is not conducted away as rapidly, so your skin doesn’t cool as much. You think (incorrectly) that the glass is cooler than the plastic. In fact, they are the same temperature. The glass, however, cools your warm skin faster than the plastic does.
Solid, Liquid, Gas, and Plasma
Aristotle said there were only four elements: air, earth, water, and fire. In retrospect that sounds silly – unless what he was really referring to was what we now call the “states” of matter. Air is the most common gas, earth the solid, water the liquid, and fire is the most common plasma.
At low temperatures, the shaking of molecules in a substance is low, and the molecules tend to stick together in a rigid form we call a solid. When the substance gets hotter, the molecular motion increases to the point that the bonds to nearby molecules are weakened. The molecules still stick, but they can now slip past each other. When they reach this point, we say we have a liquid.
The most remarkable thing about this change is that it happens so abruptly. Water at 31 F is a solid, and water at 33 F is a liquid. The change from solid to liquid is called a change in “phase.”
As we continue to heat the water, the molecular shaking increases, but until the temperature reaches 212 F (= 100 C), the molecules slip but they still stick. At 212 F, the shaking is finally enough to overcome the attractive forces between the molecules, and they break apart. This is the phenomenon called boiling, and the escaped molecules are now a gas.
Even below 212 F, some molecules will have sufficient energy to break away. This happens because not all the molecules have the same energy; some are shaking faster, and some slower. The faster ones are the ones that can break away. When they do that, and leave the surface, then the molecules left behind are the slower, colder ones. That’s why evaporation makes the liquid cooler--it’s just because the hotter molecules are leaving.
At even hotter temperatures, collisions between the molecules are sufficient to break them apart into individual atoms. If the atoms are themselves broken apart, so that electrons are knocked off their surfaces, then we call the gas a plasma.[19] A plasma consists of electrons with negative electric charge (see Chapter 4 “Nuclei and Radioactivity”). The remaining atom fragment, which has a net positive charge, is called an ion. A plasma has no net electric charge because it is a mixture of negatively charged electrons and positively charged ions.
Here’s an important fact. The temperature at which a solid melts (e.g. 32 F for ice) is the same as the temperature at which the liquid (water, in this case) freezes. Similarly, water boils at 212 F. If you have a hot gas, and cool it, it will begin to condense, that is, turn into a liquid, when you lower the temperature to 212. This symmetric behavior seems obvious to some people, and surprising to others.
Solids, liquids, and gases are commonplace, but many people think plasmas are exotic. They are more common than you might guess. If gases are hot enough that the collisions knock electrons off the molecules, then the result is a plasma. A candle flame is a plasma. The gas inside a fluorescent light bulb is a plasma. The surface of the Sun is a plasma. A bolt of lightning is largely plasma.
Exploding TNT
Let’s think again about what happens when TNT (trinitrotoluene) explodes. According to the energy table in Chapter 1, the chemical energy that is released is 0.65 Calories per gram of TNT. When TNT explodes, it suddenly converts 0.65 Cal/g into heat. This new thermal energy is much greater than its prior thermal energy, which amounted to only 0.004 Cal/g.[20] In other words, after the explosion, the internal kinetic energy increases by a factor of 167. If the molecules didn’t break apart (they do--and that complicates it a little), the absolute temperature would suddenly become 167 times greater than the prior temperature (300K). That makes the temperature 167 x 300 = 50,000 K. Note that if we convert back to Celsius, C= 50,000 –273 ≈ 50,000 C (rounding to the nearest 1000).
50,000 C is very hot, much hotter than the surface of the Sun (which is about 6000 C). Nothing is a solid at 50,000 C. The forces between the molecules are not strong enough to hold them together. That means that our gram of TNT is suddenly converted into a very hot gas, perhaps even into a plasma.
What will that hot gas do? Even at normal room temperatures, gases take typically 1000 times the volume of a solid. So just the fact that it turns into a gas makes it expand by a factor of 1000. But since it is hot, it expands even more--by another factor of 167 (the ratio of temperatures before and after). We’ll discuss the extra factor of 167 in the next section. But for now, accept that figure. Put that factor on top of the factor of 1000, and we get a total expansion in volume of 167,000. (This is only a rough estimate.)
To summarize, here is our picture of what happens when TNT explodes: The solid material is suddenly converted into a hot gas. The hot gas expands rapidly until its volume goes up by a factor of 167,000. The expanding gas pushes everything out of the way. Any nearby material picks up the velocity of the gas. Terrorists typically surround the explosive with a pipe, or pieces of metal (e.g. nails). When the metal fragments fly out at high speed, they are what do the most harm.[21]
Gas Temperature and Pressure:
The “Ideal Gas Law”
Why did the heated gas in the last section expand by an additional factor of 167? It helps to understand the difference between a solid and a gas. In a solid, the atoms bounce back and forth, but never leave their relative positions. As the solid gets hot, this added bouncing makes the solid expand. But when the energy of the molecules becomes sufficiently great, the atoms push their way out. At high temperature the molecules no longer stay in the same place, but move much more freely. They bump into other molecules, and they bounce against any walls in the containers that they are in. The bouncing tends to push the walls outward. A force must be applied to the walls to keep them from moving.
The pressure of a gas is defined as the force it exerts on one square meter of area. The key result is:
P = constant x TK
This equation is part of the “ideal gas law.” It is called ideal because most real gases deviate from it a little bit, yet it is usually a good approximation.[22]
The importance of this law is as follows: if you double the absolute temperature, you double the pressure of the gas. If you raise the absolute temperature by a factor of 167 (as in our TNT example) then the pressure increases by a factor of 167. That’s why hot gases exert so much pressure.
Automobile airbags
The airbags that are used to protect you during an automobile crash are balloons that inflate very rapidly--in a thousandth of a second--in between the time that the crash is detected by the automobile electronics and the time that your head would smash into the windshield. How can you fill a balloon that rapidly? The answer is, naturally, with an explosion. Airbags contain about 50 to 200 g of an explosive called sodium azide. Its molecules consist of 1 atom of sodium and 3 of nitrogen; it has the chemical formula NaN3. When triggered by an electric pulse, it explodes into sodium metal and nitrogen gas. The released gas inflates the balloon.
Leidenfrost layers, sautéing and firewalking
Have you ever seen a drop of water land on a hot sauce pan? It seems to float above the surface and move about as if there were no friction. If you have never seen this, try it. Put on a pair of glasses to protect your eyes. You’ll see the drop sizzle, and then float just above the surface of the pan.
This happens because the rapid heating of the water turns it into a gas and pushes the drop away from contact with the pan. The gas has very little friction, and so the droplet moves over the surface. The gas also conducts heat very poorly, since it is a thousand times less dense than the water (so there are 1000 times fewer molecules present to carry the kinetic energy from the sauce pan to the water).
The thin layer of gas that insulates the drop of water is called a “Leidenfrost layer” after Josef Leidenfrost, the scientist who, in the 16th century, was the first to understand why water droplets floated on hot pans.
For a class demonstration, this effect is easily demonstrated with liquid nitrogen. Nitrogen is a gas that composes approximately 79% of air. It turns to liquid when cooled to 77 K = –196 C = –321 F. Pour some on a table, and watch the little droplets of liquid nitrogen scoot over the table top, suspended on thin layers of nitrogen gas.[23]
Some people believe that the Leidenfrost effect can explain “firewalking,” the ability of people to walk barefoot over hot coals without burning their feet. If the skin of your foot is moist (e.g. from sweat) and you step on a hot coal, the water is very rapidly boiled into a thin layer of gas. The water vapor from the sweat has a temperature of 100 C; it penetrates into the hot coals, and prevents the much hotter gases from the interior from reaching the feet. Although the hot water vapor is hot, it is also a poor conductor of heat, so it doesn’t heat the foot very quickly.
Look up firewalking on the Internet; you will find lots of commercial organizations that will lead you through a firewalking ritual as part of a self-improvement and confidence-building program. (If you can walk on fire without being burned, you can do anything….) But I don’t recommend you try walking on hot coals without professional supervision. I would guess that the professionals first make sure your feet are adequately damp (e.g. from walking on moist sand near the sea) and they use special coals. Here is something you can try with relative safety: Next time you are at the beach on a hot day, and the sand is too hot to walk on comfortably, wet your feet, and try again. You’ll discover that you can walk a few tens of meters before the sand becomes unbearably hot. Of course, the temperature of the sand didn’t change, just the flow of energy into your feet. And be careful, even hot sand can scald your feet. If you never get to leave the city, you can try the same thing on a hot sidewalk. But carry some sandals with you in case your feet begin to burn.
Automobile: explosions under the hood
We’ve talked about turning energy (e.g. energy of motion) into heat, but can we do the opposite? There is a huge amount of energy hidden as heat. Can it be turned into useful energy?
Yes. Exploding TNT turns chemical energy into heat, the heat causes the material to turn into hot gas, and the expanding hot gas can rip apart rock. That counts as useful work.
We can also tame this process to do some more gentle work, like running an automobile. Gasoline and air are injected into a chamber called a cylinder (because of its shape) making an explosive mixture. A spark (from the spark plug) ignites the mixture, it explodes,[24] and the mixture turns into a hot gas. The high pressure from this gas pushes a piston, which in turn pushes a series of gears that turn the wheels.
The explosions in an automobile are generally kept small so they won’t rip the engine apart. Your car probably has 4 to 8 cylinders, and these are run in sequence to provide a fast series of bursts that approximate continuous power. If you would really like to get a sense of how a gasoline engine works, to see the animated image take a look at http://auto.howstuffworks.com/engine1.htm When run by a web browser, it shows the cycles of the engine. Go to the Web site to see it in operation. At the top of this web image is a spark plug. Gas and air are introduced through the valves; the spark plug ignites the mixture, forcing the piston down in the cylinder. At the end of its motion, another valve is opened; the cylinder moves upward (carried by the momentum of an attached flywheel) to expel the burned gases, and the cycle repeats.
Heat Engines
Any engine that runs by turning heat into mechanic motion is called a “heat engine.” An automobile engine is a heat engine; so is a locomotive steam engine, and a diesel. Nuclear submarines and nuclear ships (some of our aircraft carriers are nuclear) are also run by a heat engine. Nuclear power is used to heat water to steam, and the steam is run through a turbine (a fancy fan) to make it spin. The spinning motion is conveyed to the propeller to push the submarine (or ship) forward. We’ll talk more about creating heat from nuclear energy in Chapter 5.
What kind of engine is not a heat engine? Think for a moment and see what you can think of. I’ll put some examples in the footnote to make it easy to not peek at the answer until you’ve thought about it.[25]
Wasted Energy
In an automobile engine, the chemical energy from the gasoline and air mixture is turned into heat, and the pressure from the hot gas pushes the piston. But not all of the energy turns into this useful work. Some of the heat is conducted away to the outside air and is “wasted.” For typical automobiles, only about 20% to 30% of the chemical energy is converted into useful propulsion.[26] The rest is wasted--in the form of heat that escapes, or has to be removed. In fact, gasoline engines waste so much energy that special cooling systems are built-in to get rid of the wasted heat. That is what the “radiator” in the front of the car does, it cools water by letting air blow by it, and then uses the cool water to remove waste heat from the engine (so it doesn’t “overheat”), and then sends the hot water back to the radiator to cool off.[27]
It is possible to use the energy more efficiently, but there are surprising limits. As we will see in the next section, there are limits to how well a heat engine can perform.
Limited Efficiency of Heat Engines
Here is a puzzle: The thermal energy of water at room temperature is about 0.04 Cal/g. That is small, but it is 5 times as much as in a battery. And water is cheap. Why not use the thermal energy in water as a fuel?
It turns out that there is a very fundamental theorem that limits how much of such heat can be turned into useful energy (e.g. kinetic or potential energy). This theorem was one of the greatest achievements of the theory of heat. To understand this theorem, you first have to realize that heat can be extracted (turned into useful energy) only when it is flowing from a hot region to a cold region. For example, when gasoline burns, it is hotter than the surrounding air, and that allows it to expand and push against a piston. If the surrounding air were just as hot as the exploded gasoline, it would have a similar pressure and the piston would not move. Heat engines depend on such a temperature difference to do their work.
Let the hot temperature (e.g. of the exploded gasoline) be THOT (in degrees Kelvin) and the temperature of the gas after it has been cooled be TCOLD. The amazing theorem is that the efficiency of the engine will be given by the following equation:
Efficiency is less than or equal to
1 – (TCOLD/THOT)
Perfect efficiency is 1 (i.e. 100%). Thus, for example, if the gasoline explodes at 1000 K, and is cooled to 500 K before it exhausts from the cylinder, then the efficiency of the engine will be less than or equal to 1-(500/1000) = 0.5 = 50%.
This is a remarkably simple rule, and it is always true when trying to extract energy from heat. It is not relevant for batteries or solar cells that extract energy directly from chemicals or from light. But it shows why heat engines, to be efficient, must be hot.
Let’s go back to the puzzle: Why not extract heat energy from room-temperature water? Imagine a boat that scoops water out of the sea, extracts the heat, uses it to run the propeller, turning the water into ice. The ice can then be thrown overboard. This would be very nice. Let’s calculate the efficiency for such an engine. Since the boat is at room temperature, and that is the same (we assume) as the temperature of the water, then TC and TH are equal. Then the efficiency is less than or equal to 1 – (300/300) = 0. So the efficiency is zero.
You need a temperature difference in order to extract any useful energy from heat. You cannot extract heat from a single object and turn it into useful energy unless there is something colder that you can use. This fact is so important that it has been given another fancy name: the second law of thermodynamics.
It is not necessary for you to memorize the efficiency equation. But you should know that to have high efficiency, you have to have large temperature differences (e.g. between the hot exploded gasoline and the cool outdoors). If temperature differences are small, then you cannot extract very much useful energy from heat.
Volkswagen “Bug” and the efficiency equation
In the 1960s, the Volkswagen company introduced the car that was commonly known as the “Beetle” or the “Bug.” At a time when other cars averaged 6 to 15 mi/g, it got 30 mi/g. That was, in part, because it was little. But it also ran its engine at a higher temperature, a temperature at which higher efficiency was obtained, according to the efficiency equation. If TH gets very large, then the ratio TC / TH gets very small, and the efficiency 1 – (TC/TH) gets close to 1, i.e. to 100% efficiency.
When I bought my first Beetle, in 1966, there was another advantage: the car was believed to produce very little air pollution. That’s because at the high temperature of the engine, virtually all the carbon particles in the exhaust were burned into carbon dioxide. The result was the virtual total absence of “smoke” at the back exhaust. But a few decades later, people began to worry about other kinds of pollution, in particular, nitrous oxides, NO and NO. These two gases, referred collectively as NOx, were not considered pollution in 1966! It turns out that at high temperatures, ordinary air (N2 and O2) react chemically to form nitrous oxide, and nitrous oxide is more important in the formation of smog than carbon particles. The Beetle produced huge amounts of nitrous oxides because of its high engine temperature. The nitrous oxide production could not be reduced without reducing the temperature of the engine, and if they did that, then the efficiency of the engine would go down. When new legislation limited the nitrous oxides allowed from new cars, the old Bug was phased out. The “new” Volkswagen Beetle (no longer in manufacture) uses a water-cooled engine that operates at low temperatures to avoid making nitrous oxides, but as a result, it is not as efficient as it could be.
Refrigerators and Heat Pumps
A heat engine requires a temperature difference, something hot (to provide the energy) and something cold (for the heat to flow to). In an automobile engine this is created by burning (exploding) gasoline. As the hot gas expands, it does useful work (i.e. it turns the wheels of the car). It is possible to reverse this process: to take mechanical motion, and use it to create a temperature difference. The device that does this is called a refrigerator or a “heat pump.”
A typical refrigerator works by using a mechanical force to reduce the pressure in a chamber. Then the gas law equation, P = constant x T, implies that the temperature of the gas will decrease. That cool gas can be used to freeze ice, or just to cool a room. That is how refrigerators and air conditioners work.
The mechanical force that reduces the pressure must push the piston against the room air pressure. This motion slightly heats the air. So in a refrigerator, not only is one side being cooled, but the other side is being heated. Energy is conserved, so any heat that leaves the refrigerator must result in energy transferred elsewhere, usually to the surrounding air in the room. Thus, refrigerators heat the rooms that they are in. Air conditioners are designed cool a room and put the extra heat outside. That’s why air conditioners must be placed in windows or other locations with access to the outside. You can think of an air conditioner as a device that uses mechanical motion (usually from an electric motor) to pump heat from inside the room (where it is warm) to the outside (where it is cold).
The reverse also works. On a winter day, you can take an air conditioner, install it backwards, and use it to pump heat energy from the cold outdoors into a warm room. That means it takes some of the thermal energy out of the cold outside air – making it even colder – and brings that energy indoors to make the indoors warmer. When used in this fashion, the device is usually called a heat pump. Heat pumps are widely used in cold parts of the United States. It is exactly the same as using an air conditioner in reverse, to make the outside colder and the inside warmer.
Here is a puzzle with a surprising answer. Suppose you have a gallon of fuel, and a cold house. What is the best way to heat your home? You can burn the fuel, and use the heat produced. But here is a much better way: use the fuel in a heat engine, and use the mechanical motion that is produced to run a heat pump. The heat pump will extract heat from the cold outdoors, and pump it into the room. It turns out that the heat pump method will put typically three to six times more heat into your room than if you were to just burn the fuel and use the heat emitted. This factor over burning is called the “coefficient of performance” or COP.
Does that mean that we are wasting fuel when we heat our homes by burning fuel (gas, coal or wood) instead of using that fuel to run a heat engine/heat pump system? The surprising answer is yes. But an engine/pump system is more complicated and its costs more. It generally isn’t used unless the outside temperature is very cold, since otherwise it is cheaper to buy more fuel than it is to buy the expensive engine/pump system. But as we run out of fossil fuel, and it becomes more expensive, we can expect to see much wider use of engine/pump heating systems.
Look back now at the fourth “quandary” listed in the opening paragraph of this chapter.
Laws of Thermodynamics
Here is a complete list of the laws of thermodynamics.
|
0th Law: |
objects in contact tend to reach the same temperature |
|
1st Law: |
energy is conserved (if you consider all the forms,
including heat) |
|
2nd Law: |
you can’t extract heat energy without a temperature
difference |
|
3rd Law: |
nothing can reach the temperature of absolute zero |
The second law can also be understood as the fact that all objects in contact with each other tend towards “equilibrium,” i.e. they all tend towards the same temperature. A famous consequence of the second law is that whenever heat flows, the total “disorder” in the Universe tends to increase. The third law is plausible, since it is hard to remove heat from an object without having something that is colder, and so it is difficult to remove heat from any object that is close to absolute zero.
It is not necessary that you memorize this numbered list of laws. It is far more important that you know the facts, i.e. that objects in contact tend to reach the same temperature, that energy is conserved, etc.
Heat flow: Conduction, Convection, and Radiation
Heat energy moves from one place to another in three ways, called conduction, convection, and radiation.
Conduction: energy flows by contact. We discussed this earlier when we talked about touching glass vs. plastic. Hot molecules transfer energy to cooler ones by direct contact. A good conductor is something that transfers heat rapidly from one molecule to the next. Metals are usually good conductors, as is glass. Plastic is a poor conductor. If you want to insulate something from heat, you use a poor conductor. If you want a pan that is heated at one point to distribute that heat over its whole surface, you make it out of a good conductor (e.g. aluminum or copper).
Convection: energy is carried by a moving material, usually a gas or a liquid. When the hot material reaches some cold things, it usually transfers its energy by contact, i.e. by conduction. Example: an electric heater in your room warms the nearby air (by conduction). That air then moves throughout the room (convection), warming things that it touches (by conduction). A fan can help convection. Hot air also tends to rise (see Chapter 3) and that starts the air in a room circulating on its own; that’s called “natural convection.” A convection oven uses circulating hot air to heat food.
Radiation: energy moves through empty space, carried by (possibly invisible) light. When you stand in sunlight, you are warmed by radiation from the Sun. When you stand in front of an infrared heat lamp, you are warmed by the invisible infrared radiation. (We’ll discuss such invisible light in much more detail in Chapter 9.) A microwave oven cooks by radiation. Microwaves penetrate into food, so they will cook the insides of some foods as rapidly as they cook the outsides.
The word radiation is used for virtually any energy that flows through space. This includes nuclear radiation (which can cause cancer; see Chapter 4), visible light, ultraviolet light (which can cause sunburn, see Chapter 9), and microwaves.
Optional: Entropy and Disorder
I mentioned earlier that we can quantify the concept of “disorder” into a number called the entropy, and that when heat flows, the net entropy of the Universe tends to increase. This subject receives a great deal of attention from philosophers, and so it is worth a bit of further discussion.
When entropy changes due to heat flow, the calculation is simple: When heat flows into an object, its numerical increase in entropy is Q/T, where Q is the amount of heat (usually measured in joules) and T is the temperature. When heat leaves an object, the entropy of that object decreases by Q/T.
When heat flows from a hot object (with temperature TH) to a cold object (which has temperature TC), the total change in entropy is:
total change in entropy = Q/TC – Q/TH
The first term will always be bigger than the second one (since TC is smaller than TH) and so the total entropy will increase. This is the deep meaning of the fact that the entropy of the Universe is increasing. The Universe is becoming more and more disordered.
Disorder can also increase without heat flow. For example, if you burst a balloon, then the atoms are no longer confined into a small region, but have spread out through the atmosphere. This kind of disorder can be included in with the other.
It is important to realize that the entropy of an object can go up or down; it is the total entropy of the Universe that is always increasing. My goal with this book is to decrease the entropy in your brain. (That is a fancy way of saying that I hope your learn something.) As you learn, you will radiate heat, and that will increase the entropy of the world around you. The net entropy of the Universe goes up, but I hope that your own entropy goes down.
When an object cools off, its entropy goes down, but the heating of the surroundings more than makes up for that, so the total entropy of the Universe increases. The entropy of the Earth is decreasing with time, as is the entropy of the Sun. The Sun is emitting visible light; the Earth is emitting infrared light (see Chapter 9) and as a result, the total entropy of the Universe goes up.
Some philosophers (and some physicists) have argued that the increase in entropy of the Universe is what determines the direction of time, i.e. why we remember the past and not the future. (That really is a deep question, not at trivial as it sounds.) But it can also be argued that it is the local decrease in entropy (i.e. when we learn things) that gives us the sense of time.
Have fun thinking about these ideas. There have been several popular books devoted to the subject. The second and third laws of thermodynamics can be reformulated to read as follows:
Second Law: The entropy of the Universe tends to increase.
Third Law: The entropy of an object goes to zero at T = 0 K.
Understanding how these reformulations are equivalent to the original statements is part of the advanced study of thermodynamics.
Chapter Review
Atoms, the basic constituents of matter, are about 10-8 cm in diameter. There are about 50,000 of them in the diameter of a red blood cell, which is about the smallest object visible with visible light. Heat is the shaking of molecules, the fact that they have kinetic energy. The velocity of shaking is comparable to the velocity of sound, about 1000 ft/s = 330 m/s. For solids, the atoms remain in the same location despite this violent shaking. The effects of shaking can be observed in Brownian motion. It also makes itself evident in electronic noise such as hiss.
If two objects have the same temperature, then the average kinetic energy of the molecules in the two objects is the same. However, the speed of the molecules is not equal. Equal kinetic energies, an object with light molecules will have the faster ones. The lightest molecules of all, those of hydrogen gas (H2), move so fast that most of them have escaped the gravity of the Earth and are no longer present in the atmosphere.
Temperature can be measured on the Fahrenheit scale, or the Celsius (Centigrade) scale. But more useful for physics is the absolute or Kelvin scale, for which 0 K corresponds to a kinetic energy per molecule of zero. A change of 1 K is equal to a change of 1 C is equal to a change of 9/5 F ≈ 2 F.
Most objects expand when they get warm, by an amount typically by a part per 1000 to a part per 100,000 for each degree C. This is used for thermometers, but it also results in sidewalk cracks, and it could cause substantial sea level rise if global warming warms the oceans.
Heat can be transferred by conduction, since atoms in contact with others can share their kinetic energy. Gases expand when they get hot. A good approximation is the “ideal gas law” which says that the gas pressure is proportional to the absolute temperature. Heat can also flow from radiation and from convection.
An explosion occurs when an object gains so much energy that it becomes a very hot gas. It is the high pressure of the gas and the resulting rapid expansion that make up an explosion. Such explosions also occur in the cylinders of internal combustion engines, and we use them to supply energy to our automobiles.
Energy converted to heat can not always be converted back efficiently. The limit is the efficiency equation, efficiency Ł 1 – TC/TH. Such heat is often considered wasted, and may result in the ultimate “cold death” of the Universe.
The four major laws of thermodynamics are: (0) objects in contact tend to reach the same temperature, (1) energy is conserved, (2) extracting useful energy from heat requires a temperature difference, and (3) no object can be made to have T = 0 K.
Energy can be used to extract heat from an object. That is the basic principle of the refrigerator, the air conditioner, and the heat pump.
Entropy is a measure of the disorder in molecules. Warm objects, by their shaking, have more disorder, and therefore higher entropy. Whenever heat flows, the entropy of the universe increases, although the entropy of an object (such as your brain) can decrease. Indeed it does, when you learn something.
Question for discussion
If you go to a high altitude, the temperature of the air is usually lower. What do you think that does to the sound velocity? (This issue will turn out to be important when we discuss UFOs, in Chapter 7.)
Essay questions
1. Read an article that involves physics or technology that appeared in the last week or two. (You can usually find one in The New York Times in the “Tuesday Science Section.”) Describe the article in one to three paragraphs, with emphasis on the technological aspects (not on business or political aspects). If you don’t understand the article, then you can get full credit by listing the things that you don’t understand. For each of these items, state whether you think the writer understood them.
2. Describe in a page what aspects of this chapter you think are most important. What would you tell your friends, parents, or children are the key points that are important for future presidents or just good citizens.
3. When objects are heated, they usually expand. (There are some exceptions.) Explain why, and give examples that illustrate how this behavior causes problem, and how it can be usefully applied.
4. Describe what is meant by an “explosion” and explain what is going on in terms of atoms and molecules.
5. Give examples of “small” explosions that have useful purposes, particularly explosions that the average person doesn’t even realize are explosions.
6. Estimate how many atoms there are in a sheet of paper. (This question is deliberately vague. Take the paper size to be anything reasonable. Use the average size of an atom as described in the text.)
7. Discuss the efficiency equation. What are its implications for automobile engines?
8. If you double the temperature of a gas, what happens to the velocities? Do they double? (The answer is no.) Work out how much the velocity increases.
Internet research questions
1. Look up “firewalking” on the Internet. See if you can find organizations that offer firewalking training, and also see if you can find sites that explain how firewalking is possible. Describe what you find.
2. What are the most common elements in the human body? Compare them to those in the Earth’s crust. Which do you consider the biggest surprises?
3. Look up “heat engines” on the Internet. There will be a lot of technical discussion meant for engineers. Can you find any novel heat engines? Look up “nitinol engine.” Can you find heat engines that claim to work on small temperature differences? Do they discuss the poor efficiency that you get with small temperature differences (from the efficiency equation)?
4. Look up “heat pump” on the Internet. What can you find out about the coefficient of performance? What is the cost? Would a heat pump be a good investment in the area you live? (Don’t guess. Work out some numbers.)
5. Why is it warmer in the summer than in the winter? Is the Earth farther away from the Sun? (No.) Does the intensity of the Sun change? (No.) Why does Australia have winter when we have summer?
Multiple-choice questions
class=Section2>
1. If you double the energy content of a kilogram of gas, the temperature of the gas (measured on the absolute K scale):
( ) is unchanged
( ) increases by the square-root of 2
( ) doubles
( ) is multiplied by 4
2. About how fast are molecules in air moving?
( ) 1000 ft/s
( ) the speed of light
( ) 9.8 m/s
( ) 9.8 cm/s
3. Temperature is the measure of
( ) average momentum
( ) average kinetic energy
( ) average velocity
( ) average total energy
4. In a bucket of water, the instantaneous speed of the molecules is closest to
( ) zero, since they don’t move
( ) whatever speed the wood is moving
( ) approximately 1.7 m/s
( ) approximately 1000 ft/s
( ) approximately 186,000 miles per sec
5. A refrigerator
operating in a room
( ) warms the room
( ) cools the room
( ) has no effect on the room
( ) removes water vapor from the room
6.
Molecular motion stops at
( ) 0 K
( ) 0 C
( ) 0 F
( ) 32 F
7.
Water melts at
( ) 0 K
( ) 0 C
( ) 0 F
( ) 100 C
8.
A gas heater warms a room mostly through
( ) convection
( ) conduction
( ) radiation
( ) depletion
9. A table has the same temperature as the air above it. That means that the molecules in the air and in the table have
( ) The same average velocity
( ) The same average energy
( ) The same average acceleration
( ) The same average mass
10. The velocity of sound
( ) increases when the temperature increases
( ) decreases when the temperature
increases
( ) depends on the pressure of the air
( ) is constant, independent of the
temperature
11. If a gasoline engine produces a hotter explosion, then the efficiency of the engine should
( ) be the same
( ) goes up
( ) goes down
12. A cup full of water made of plastic feels warmer than one made of glass because:
( ) plastic is warmed by water
( ) plastic conducts heat less than glass
( ) plastic conducts heat better than glass
( ) plastic dissolves in water
13. The atom with the fewest number of protons is
( ) helium
( ) carbon
( ) hydrogen
( ) oxygen
14. How many atoms are there if you move across a human hair? About
( ) 25
( ) 125,000
( ) 273,000,000,000
( ) 6 x1023
15. The speed of sound is approximately
( ) 1 foot per nanosecond
( ) 1 foot per second
( ) 1000 feet per second
( ) 186,000 miles per hour
16. Put hot glass in cool water. It shatters because
( ) it heats the surface water to boiling
( ) the outer surface of the glass contracts rapidly,
but the inner part doesn’t
( ) the outer surface of the glass expands rapidly
( ) the rapid conduction of heat triggers
strong vibrations
17. Sea level is rising from global warming. The main cause is
( ) expanding sea water
( ) melting glaciers
( ) expanding rock under the sea
( ) contracting earth (while the water stays constant)
18. The temperature warms by 2 C. That is approximately the same as
( ) 1 F
( ) 2 F
( ) 4 F
( ) 1/2 F
19. To heat a room using the least energy, do it by:
( ) burning natural gas (methane)
( ) using natural gas to run a heat pump
( ) burning coal
( ) burning gasoline
20. There is almost no hydrogen gas in the atmosphere because
( ) it escapes the earth’s gravity
( ) there is very little hydrogen in the oceans or land
( ) hydrogen molecules move slower than oxygen
and nitrogen
( ) hydrogen has all sunk to the earth’s core
21. At Mach 10, if all the energy of a meteorite went into heating it, its temperature would be about
( ) 300 C
( ) 6000 C
( ) 30,000 C
( ) 100,000 C
22. The New Orleans levees failed because
( ) thermal expansion broke them
( ) they were made of continuous concrete
that could not stand the pressure
( ) they had leaks at the thermal expansion
joints
( ) they were weaker at the thermal
expansion joints
23. If temperature rises by 5 C = 9 F, then the rise in sea level will be about
( ) 2 inches
( ) 4 feet
( ) 12 feet
( ) 97 feet
24. For a typical auto, the fraction of the gasoline energy wasted as heat is about (careful, this may be a trick question):
( ) 1%
( ) 10%
( ) 20%
( ) 80%
25. Temperature in the sun
( ) is always hotter than in the shade
( ) is always the same as in the shade
( ) is sometimes cooler than in the shade
( ) is not well defined
26. When material is cooled
( ) it stays the same size
( ) it contracts
( ) it expands
( ) some things contract and some expand
27. The melting temperature of an object is usually
( ) equal to its freezing point
( ) higher than the freezing point
( ) lower than the freezing point
( ) equal to the boiling point
28. Absolute zero is the temperature of
( ) frozen water
( ) the universe
( ) liquid helium
( ) nothing
29. When a liquid boils, the increase in volume (liquid to gas) is typically a factor of
( ) 10
( ) 100
( ) 1000
( ) 1,000,000
30. If the temperature of a gas in a container goes from 0 C to 300 C, the pressure will
( ) stay the same
( ) double
( ) become 300 times greater
( ) become infinite
31. Firewalking is similar to
( ) water on a saucepan
( ) the space shuttle reentry
( ) automobile air bag
( ) water skiing
32. For gasoline to explode, it requires
( ) to be mixed with oxygen
( ) to be mixed with nitrogen
( ) to be mixed with carbon dioxide
( ) no mixing needed; just a spark
33. To be more efficient, the temperature difference (between the ignited hot fuel and the cool part of the engine) should be
( ) as small as possible
( ) as large as possible
( ) it doesn’t matter
34. Heat flow through empty space (no atoms present)
( ) is impossible
( ) occurs through conduction
( ) occurs through convection
( ) occurs through radiation
35. Entropy (optional topic) measures
( ) heat
( ) temperature
( ) disorder
( ) energy